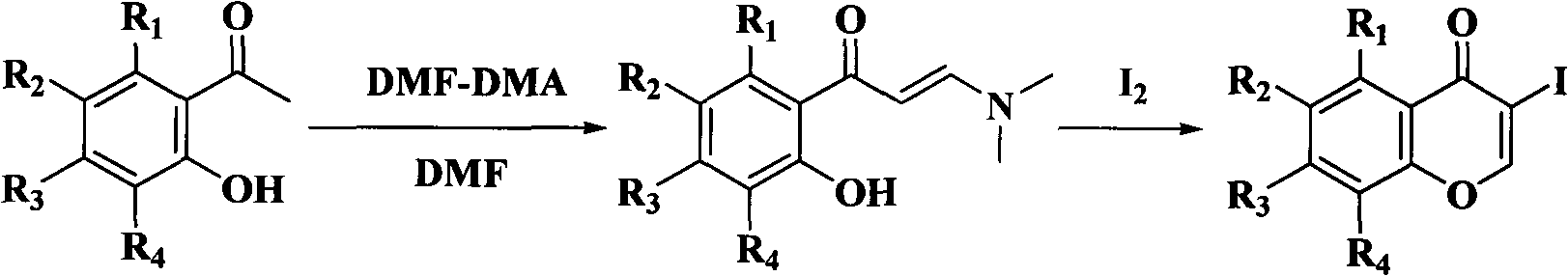
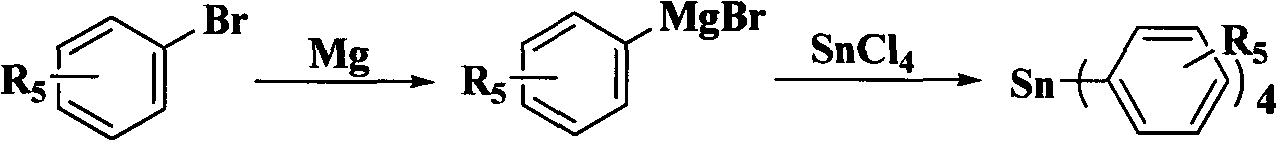
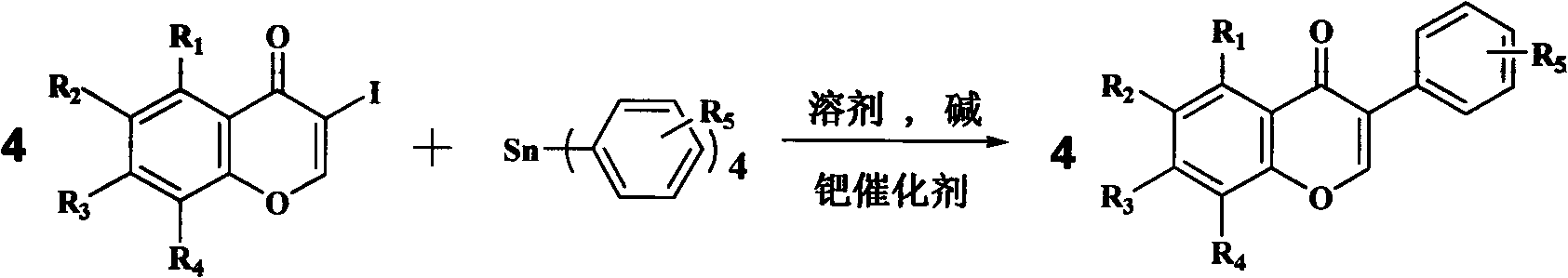
Synthesis of isoflavone compound through Stille crossed coupling reaction
A technology for isoflavones and bromoisoflavones, which is applied to isoflavone compounds and related medicines and their application fields, can solve the problems of large influence on catalyst and solvent yield, low industrial application value, complicated reaction process and the like, and achieves low production cost. , the effect of mild reaction conditions and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In this example, 3-iodochromanone and ethanol with 25 times the weight of 3-iodochromanone are added to the reaction kettle as a solvent, and after stirring evenly, add 3-iodochromanone with a molar ratio of 4:1.1 Tetraphenyltin and 10% palladium carbon with a molar ratio of 1:0.1 to chromone and sodium acetate with a molar ratio of 1:2 to 3-iodochromone, and the temperature of the reaction solution is 80° C. Make it reflux reaction, stop the reaction after 1 hour, pour an appropriate amount of column chromatography silica gel into the reaction solution after the reaction solution is cooled to room temperature, distill under reduced pressure to recover the solvent, use petroleum ether and ethyl acetate mixed solvent as eluent to pass through silica gel The pure isoflavones were separated by column chromatography gradient elution.
[0047] Adopt this example to prepare compound (1) isoflavones, after testing its physical and chemical properties are as follows:
[0048] ...
Embodiment 2
[0055] In this example, 6,8-dibromo-3-iodochromanone, 6-bromo-3-iodochromanone, 6-fluoro-3-iodochromanone, 7-methyl Oxygen-3-iodine, and acetonitrile with 20 times the weight of the corresponding chromone as a solvent, after stirring evenly, add tetraphenyltin with a mol ratio of 4: 1.3 and a chromone molar ratio of 1 : Palladium nitrate of 0.005 and sodium bicarbonate with a molar ratio of 1:4 to chromone, use a temperature regulating device to make the temperature of the reaction solution 60°C to reflux the reaction, stop after 5 hours of reaction, and cool the reaction solution to room temperature An appropriate amount of column chromatography silica gel was poured into the reaction solution, the solvent was recovered by distillation under reduced pressure, and the mixed solvent of petroleum ether and ethyl acetate was used as the eluent to separate the compounds (2) 6, 8- Dibromoisoflavone, compound (3) 6-bromoisoflavone, compound (4) 6-fluoroisoflavone, compound (5) pure ...
Embodiment 3
[0085] In this example, 3-iodochromanone, 6,8-dibromo-3-iodochromanone, 6-bromo-3-iodochromanone, 6-fluoro-3-iodochrome Proketone, 7-methoxyl-3-iodochromanone, and DMF of 10 times the weight of the corresponding chromone as a solvent, after stirring evenly, add p-methyltetraphenylene with a molar ratio of 4:1.2 to chromone base tin and tetrakis(triphenylphosphine) palladium with a molar ratio of 1:0.06 to chromone and sodium carbonate with a molar ratio of 1:8 to chromone, the temperature of the reaction solution is 100° C. Its reflux reaction, stop after 3 hours of reaction, pour an appropriate amount of column chromatography silica gel into the concentrated reaction solution to recover the solvent by distillation under reduced pressure, use petroleum ether and ethyl acetate mixed solvent as eluent, and perform gradient elution through silica gel column chromatography Separate and obtain compound (6) 4'-methyl isoflavone, compound (7) 6,8-dibromo-4'-methyl isoflavone, compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com