Nucleic acid gold-labeled rapid detection method and kit for pathogen
A detection method and pathogen technology, applied in the field of medical biology, can solve problems such as detection difficulties, biological safety hazards, unfavorable detection and large-scale general surveys
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
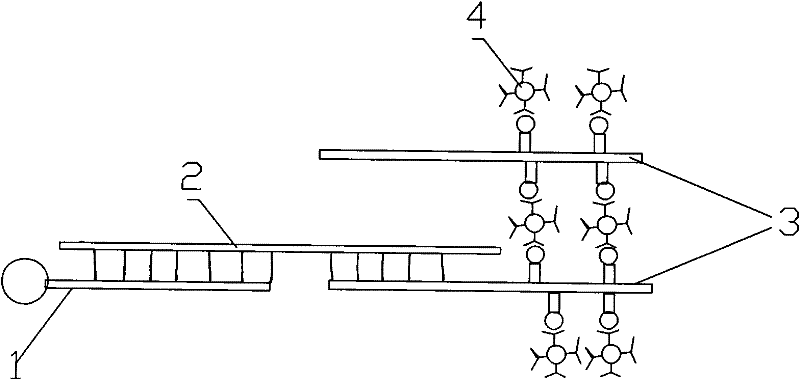
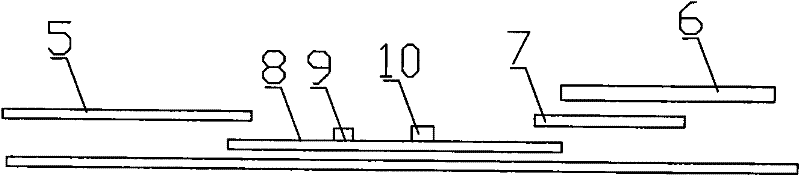
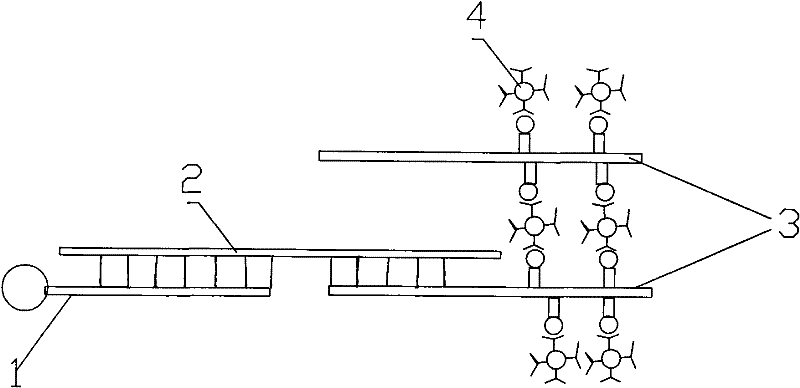
Image
Examples
Embodiment 1
[0058] Embodiment 1. The design of primer, probe
[0059] Step 1. Design of HBV nucleic acid amplification primers
[0060] According to the homologous comparison of 818 gene sequences of 8 genotypes of HBVA-H, and the analysis of their inter-type and intra-type conservation and specificity, the relatively conserved S region of the gene sequence was selected as the PCR amplification region, and two pairs were designed. PCR primer, its sequence is as follows:
[0061] Outer Primer1: 5-TCACCATATTCTTGGGAACAAGA-3
[0062] Outer Primer2: 5-CGAACCACTGAACAAATGGC-3
[0063] Inner Primer1: 5-AGRTRGGAGYGGGAGCATTCGG-3
[0064] Inner Primer2: 5-GGCACTAGTAAARTGAGCCA-3 (R is A or G; Y is C or T).
[0065] The PCR amplification of the sample is carried out by the nested PCR method, which ensures the sensitivity and specificity of the PCR amplification. Step 2. Design and labeling of HBV oligonucleotide probes
[0066] According to the homologous comparison of the gene sequences of 8 ge...
Embodiment 2
[0071] Embodiment 2. preparation detection test paper
[0072] Step 1 Preparation of specific monoclonal antibodies against hapten biotin and digoxin
[0073] 1) Immunization of animals and determination of serum antibody titers
[0074] Biotin and digoxin coupled with poly-lysine were used as antigens to immunize pure-line BALB / C female mice aged 8-12 weeks, respectively, and blood was collected from the orbit 3 days after the immunization, and enzyme-linked immunosorbent assay was used to (ELISA) was used to determine the serum antibody titer to determine the immune effect. A booster immunization was performed 3 days before fusion.
[0075] 2) Take out the spleen of the effectively immunized mouse under aseptic conditions, prepare and count the spleen cell suspension. The myeloma cells SP2 / 0 with a survival rate of not less than 95% and a good growth state were used for counting. Under the fusion-promoting effect of polyethylene glycol, it promotes the fusion of splenocy...
Embodiment 4
[0123] Embodiment 4 detection of hepatitis B virus
[0124] 1) Test sample processing and preparation
[0125]20 positive serums and 10 negative control serums confirmed to contain different hepatitis B virus loads through fluorescent quantitative PCR and five detections of hepatitis B are the specificity of the inspection method of the present invention and the designed primers and probes for testing specimen verification, each Take 200 μl of each portion, and extract viral nucleic acid with a high-purity viral nucleic acid extraction kit (High Pure viral nucleic acid kit, Roche Diagnostics, Mannheim, Germany), and dissolve the extracted viral nucleic acid in 50 μl of nucleic acid-free double-distilled water at -80 Store at ℃ for later use.
[0126] 2) Nested PCR (tested PCR) amplifies the S region fragment of HBV.
[0127] 10 μl DNA template
[0128] 5μl 10×buffer
[0129] 0.4 μl 10 mM dNTPs
[0130] 10pmol primer
[0131] 1U Taq enzyme
[0132] wxya 2 O make up 50μl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com