Adsorbent for removing sulfate ions contained in water body as well as preparation and application thereof
A sulfate ion and adsorbent technology, which is applied to the adsorbent for removing sulfate ions in water bodies and the field of sulfate ion removal from acidic industrial wastewater, can solve the problems of disordered sulfur cycle, complicated preparation process, and reduced purity of chlorine gas. , to achieve the effect of multiple cycle adsorption, high desorption efficiency and short adsorption time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of polym-phenylenediamine
[0020] Dissolve 0.0925mol (10.0g) of m-phenylenediamine (mPD) in 175mL of distilled water, add it into a 250mL four-neck flask, place it in a water bath at 25°C and stir for 20min to fully mix the monomer solution; take 0.0925mol (21.1g ) Ammonium persulfate (APS) was dissolved in 55mL distilled water (1.682mol·L -1 ), gradually added dropwise to the reaction system (completed in about 20 minutes), and continued to stir for 5 hours. Suction filtration, rinse twice with distilled water, 1:1 ammonia water and distilled water respectively. The washed product was dried in an oven at 60° C. to a constant weight to obtain black poly-m-phenylenediamine powder. It was weighed and its yield calculated to be 69%.
[0021] The present invention utilizes poly-m-phenylenediamine to absorb sulfate ions in water, and the specific implementation steps are shown in the following examples.
Embodiment 2
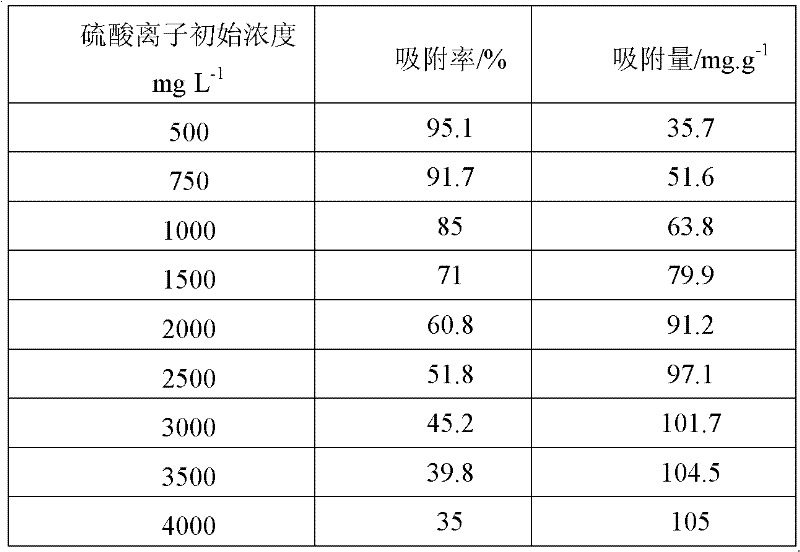
[0023] Under the constant temperature water bath condition of 30 ℃, use 200mg polymer prepared by embodiment 1 to 15mL 2000mg L -1 The sodium sulfate solution was subjected to adsorption reaction, and the reaction time was 60 minutes; the initial pH of the sulfate-containing solution was adjusted to 1.5, 1.65, 1.75, 2.0, 2.5, 3.0, 4.0, and 8, respectively. The reacted mixed solution was filtered and collected in a dry beaker, and the concentration of sulfate ions in the filtrate was determined by barium chromate spectrophotometry. Table 1 shows the adsorption rate and adsorption amount of polymers for sulfate ions at different initial pH values of the solutions. It was found that the lower the pH, the more favorable the adsorption of sulfate ions by polym-phenylenediamine. The results of this example show that the poly-m-phenylenediamine provided by the present invention can effectively treat acid wastewater such as smelting, mining, and pharmaceuticals.
[0024] Table 1 ...
Embodiment 3
[0027] Under the constant temperature water bath condition of 30 ℃, use the polymer prepared by 200mg embodiment 1 to 15mL solution initial pH is 1.75, and sulfate ion is 2000mg L -1 Sodium sulfate solution for adsorption reaction, wherein the reaction time is controlled as 1, 5, 10, 15, 30, 60min. The reacted mixed solution was filtered and collected in a dry beaker, and the concentration of sulfate ions in the filtrate was determined by barium chromate spectrophotometry. Table 2 shows the adsorption rate and adsorption amount of sulfate ions by the polymer at different reaction times.
[0028] It can be seen that the adsorption equilibrium time of poly-m-phenylenediamine is 10min. The adsorption kinetics simulation was carried out with the pseudo-second-order kinetic equation, the correlation coefficient of the simulation was 1, and the standard deviation was -1 .
[0029] Table 2
[0030] Adsorption reaction time min
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com