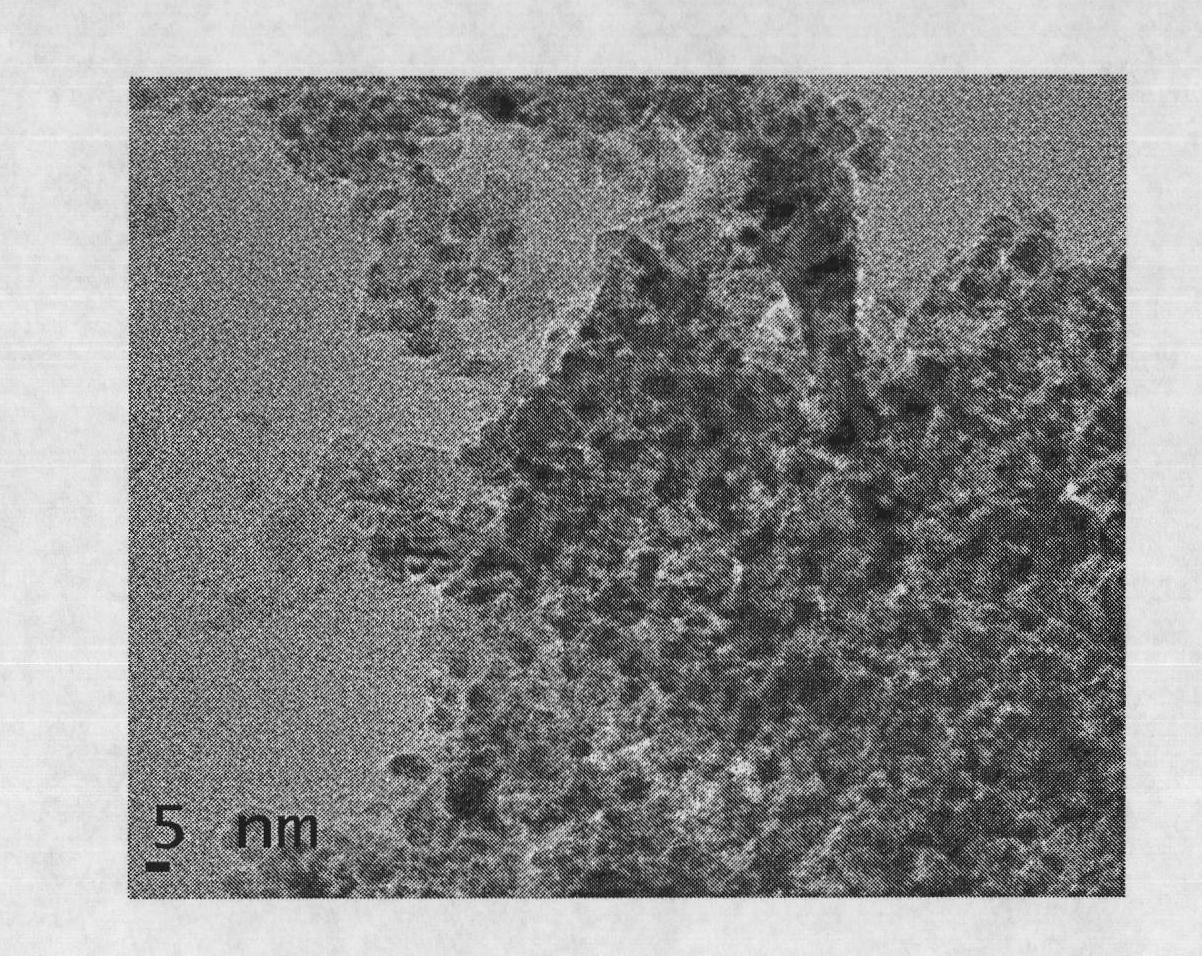
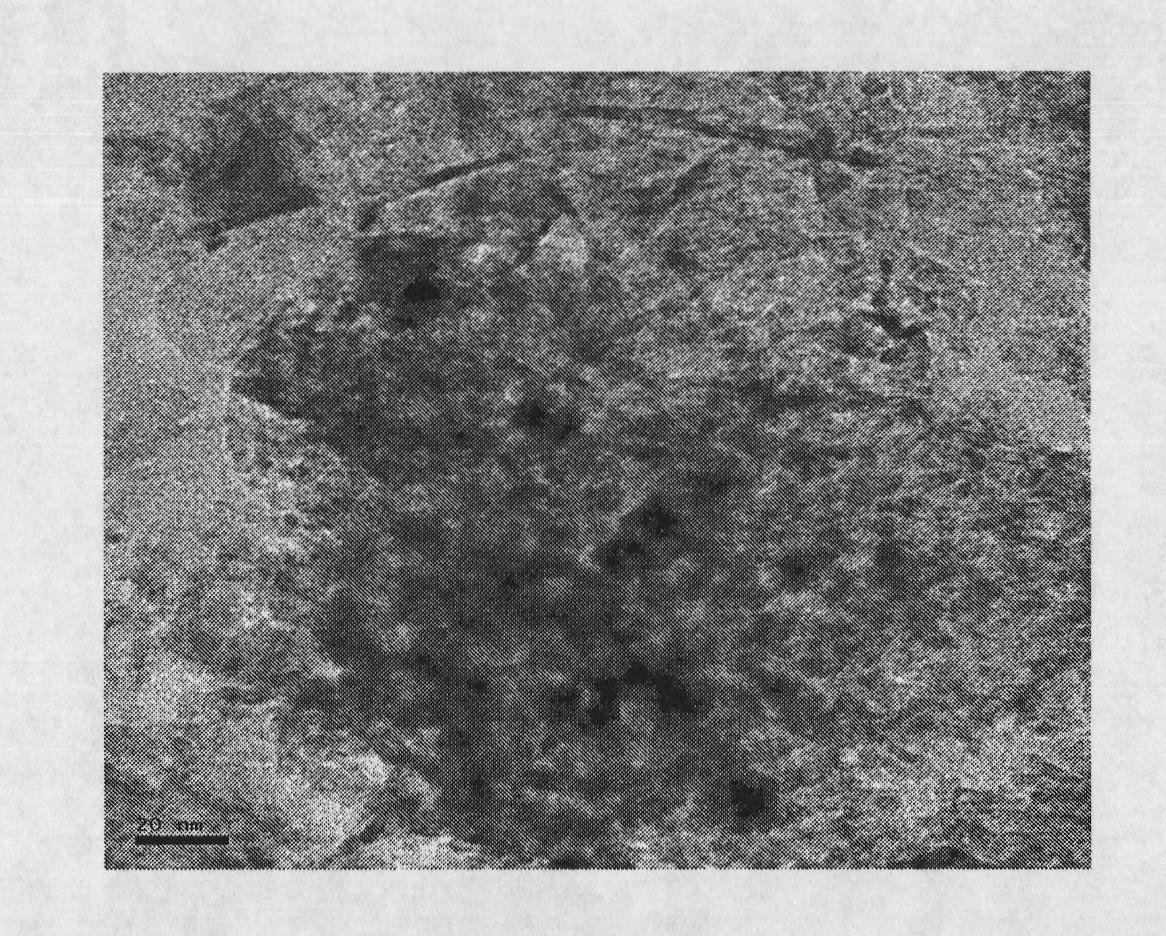
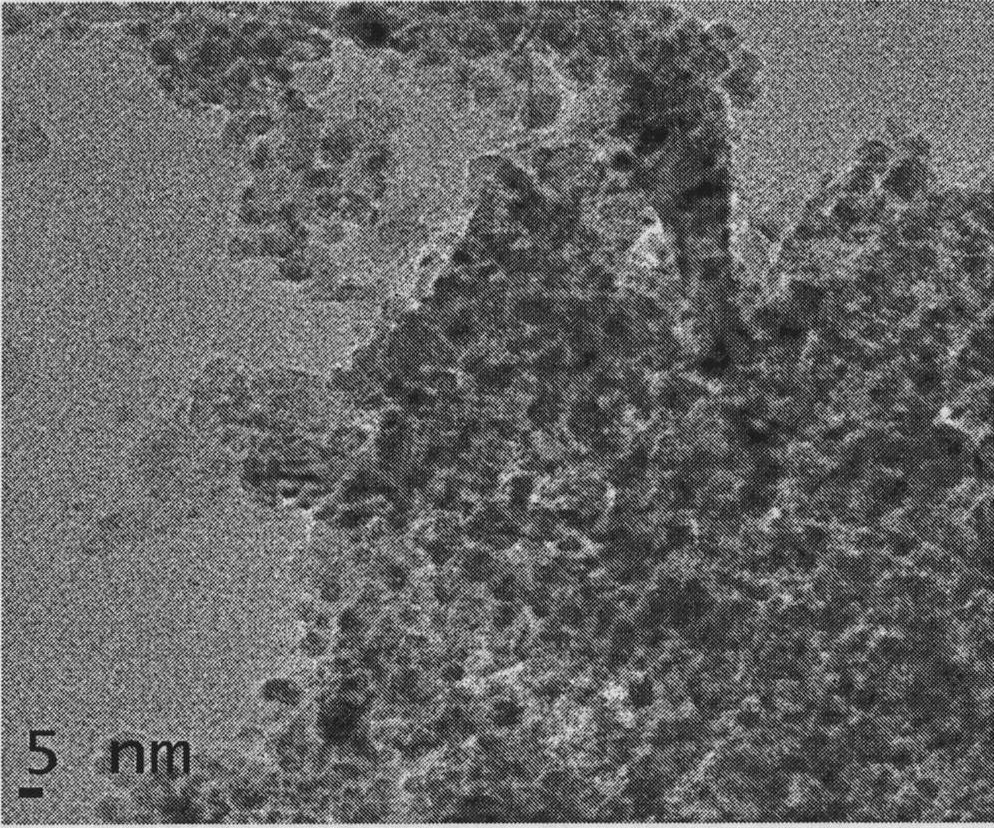
Pyrolysis gasoline selective hydrogenation catalyst and preparation method thereof
A technology for selective hydrogenation and pyrolysis of gasoline, applied in catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of affecting the catalytic performance of active components and low catalyst dispersion , Catalyst performance deterioration and other problems, to achieve the effect of shortening the preparation cycle, reducing the emission of gas pollutants, and reducing the content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O], manganese nitrate [Mn(NO 3 ) 3 ·6H 2 O], potassium nitrate (K NO 3 ) was dissolved in water to make an aqueous solution, and then the pH value of the solution was adjusted to 10 with ammonia water, and the above-mentioned alumina carrier was impregnated by an equal impregnation method, and after being baked by an infrared lamp for 20 minutes, deionized water and isopropyl 40ml of the mixed solution prepared with alcohol was poured into the carrier loaded with nickel, and after uniform dispersion, the excess solution was poured out. Use the product under vacuum 60 The Coγ radiation source was irradiated at a dose rate of 30Gy / min for 15h. The irradiated sample was dried at 120° C. for 6 hours to obtain catalyst A, wherein the nickel content was 14%, the manganese content was 1.1%, and the potassium content was 1.6%.
Embodiment 2
[0052] Nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O], ammonium molybdate [(NH 4 ) 2 Mo 7 o 24 4H 2 O], calcium nitrate [Ca(NO 3 ) 2 ] was dissolved in water to make an aqueous solution, and then the pH value of the solution was adjusted to 6 with ammonia water, and the above-mentioned alumina carrier was impregnated by an equal impregnation method, and after being baked by an infrared lamp for 20 minutes, deionized water and isopropyl 40ml of the mixed solution prepared with alcohol was poured into the carrier loaded with nickel-molybdenum-calcium, and the excess solution was poured out after uniform dispersion. Use the product under vacuum 60 The Coγ radiation source was irradiated at a dose rate of 30Gy / min for 15h. The irradiated sample was dried at 120° C. for 6 hours to obtain catalyst B, wherein the nickel content was 16%, the Mo content was 1.0%, and the calcium content was 1.2%.
Embodiment 3
[0054] Take by weighing 26.5g commercially available magnesium nitrate [Mg(NO 3 ) 2 .6H 2 O] dissolved in 30ml of deionized water, then diluted to 70ml with deionized water, weighed 100g of lanthanum-modified alumina carrier, sprayed the configured 70ml of magnesium nitrate solution on the alumina carrier, and placed in an oven at 120°C after drying Dry in medium for 24 hours, use the same steps and conditions as in Example 1 to prepare a carrier loaded with nickel-Mn-Mg-k, and after standing for 20 minutes, take deionized water and isopropanol with a volume ratio of 1:1 to prepare 40ml of the mixed solution of Ni-Mn-Mg-k was poured into the carrier loaded with Ni-Mn-Mg-k, and the excess solution was poured out after uniform dispersion. Use the product under vacuum 60 The Coγ radiation source was irradiated at a dose rate of 30Gy / min for 15h. The irradiated sample was dried at 120°C for 6h to obtain catalyst C, wherein the nickel content was 14%, the Mn content was 1.0%, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com