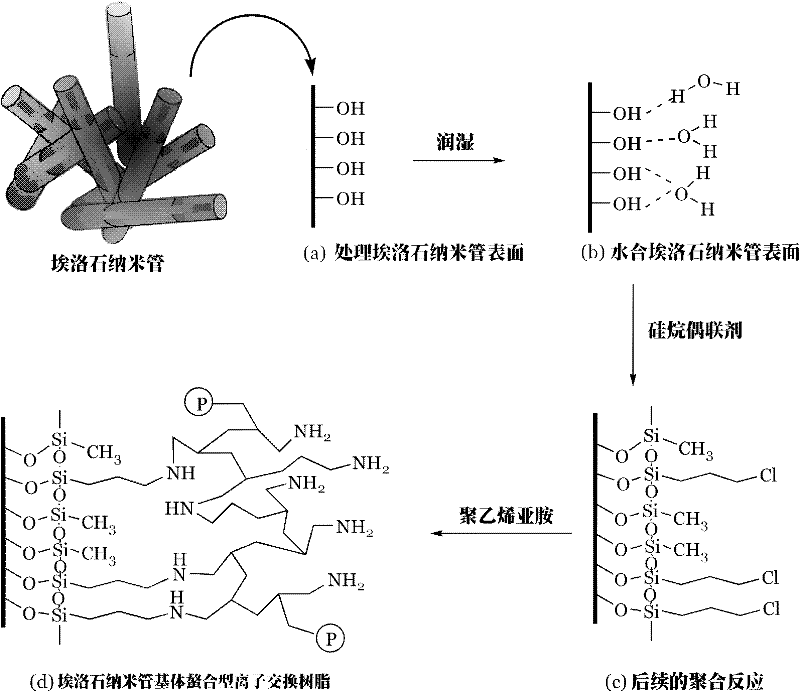
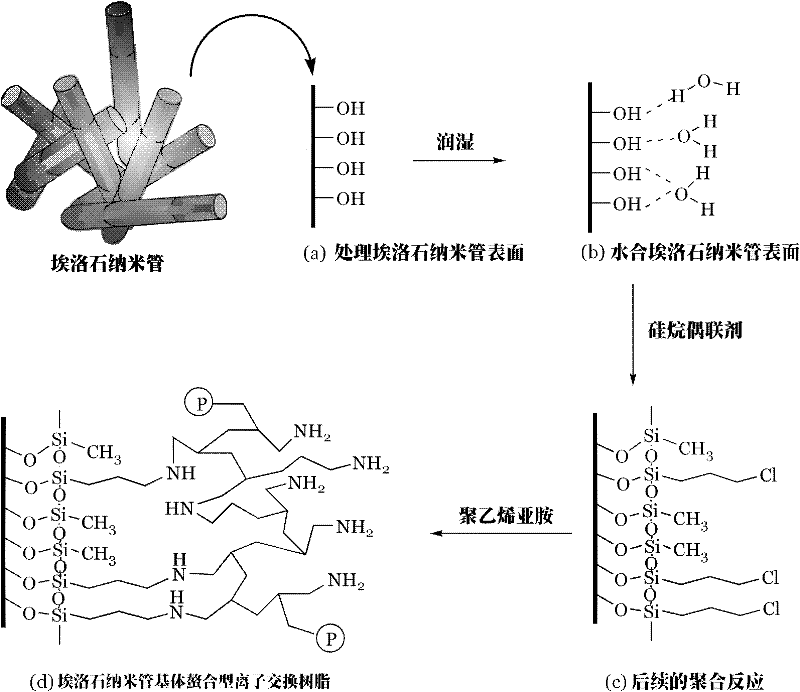
Preparation method of chelating type ion exchange resin with natural halloysite nanotube (HNT) as matrix
A halloysite nanotube and ion exchange resin technology is applied in the field of preparation of chelating ion exchange resin, which can solve the problems of complex preparation process, incapability of mass production, and high cost of carbon nanotube preparation, and achieves a short operation process. , easy operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 100 g of halloysite nanotubes into the reactor, then add 300 g of concentrated hydrochloric acid; start heating to boiling (100° C.). Stir and pickle at boiling temperature for 6 hours, and release the material when it drops to normal temperature. The released material is filtered to remove the acid solution to obtain the acidified halloysite nanotubes; then the acidified halloysite nanotubes are washed with deionized water, and the washing is stopped when the washing water shows neutrality. Separating the halloysite and water, drying the halloysite to constant weight, and obtaining dried halloysite nanotubes. The dried halloysite nanotubes (dried halloysite with constant weight) are put into a steam saturator, and the humid air of the sodium bromide saturated solution enters the water vapor saturator, so that the halloysite nanotubes A monolayer of water molecules is formed on the surface until the water content of the hydrated halloysite is 6%, and the reaction i...
Embodiment 2
[0031] Add 100g of halloysite nanotubes into the reactor, then add 300g of concentrated hydrochloric acid; start heating to boiling (100°C), stir and pickle at boiling temperature for 6 hours, and release the material when it drops to normal temperature; the released material is filtered Finally, filter out the acid solution, then wash the halloysite with deionized water, stop washing when the washing water shows neutrality, separate the halloysite and water, and dry the halloysite to constant weight. Put the dried halloysite with constant weight into a water vapor saturator, let the moist air of the sodium bromide saturated solution enter the water vapor saturator, and make the surface of the halloysite nanotubes form a monolayer of water molecules until the hydrated When the water content of halloysite is 6%, the reaction is stopped to obtain hydrated halloysite nanotubes.
[0032] Then put the hydrated halloysite nanotubes into another reactor, put 300g of n-heptane (?) and...
Embodiment 3
[0035] Except that the weight average molecular weight of polyethyleneimine is changed to 10,000, other conditions are the same as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com