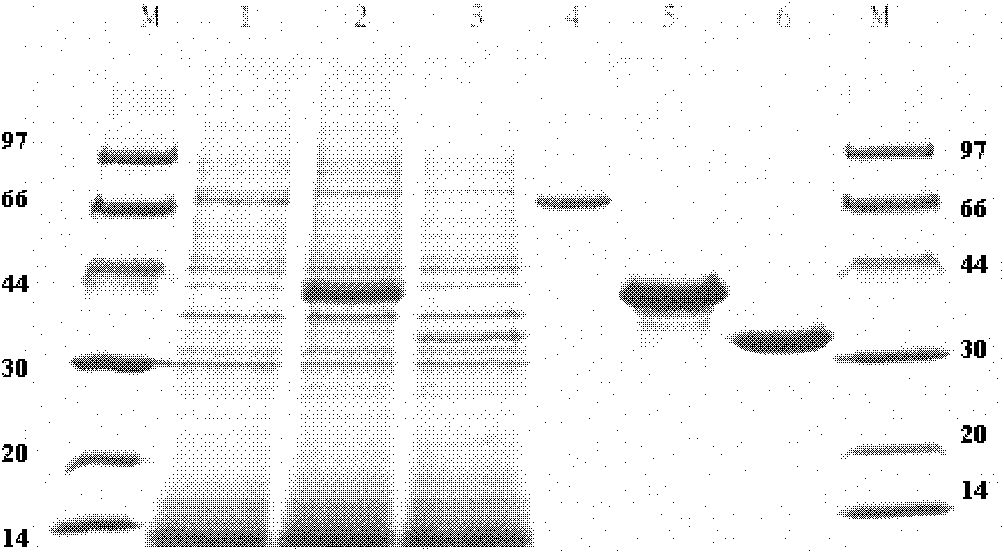Low-temperature neutral phytase PhyH with double structure domains as well as gene and application thereof
A phytase and double-structure technology, applied in the field of genetic engineering, can solve problems such as low catalytic efficiency and difficulty in meeting production needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Enzyme-producing characteristics of Bacillus sp.HJB17
[0061] Xinjiang Tianshang No. 1 glacier permafrost samples were cultured in a phytase screening medium (low phosphorus medium LPM), then diluted plates according to routine, and cultured at 4, 20, and 37°C respectively. Then the grown single colonies with transparent circles were separated and purified, and the active strains were identified. After 16S identification, the strains belonged to the genus Bacillus and were named Bacillus sp.HJB17.
[0062] The formula of low phosphorus medium LPM is as follows:
[0063] Casein peptone 1g; soybean peptone 0.45g; glucose 4g; glutamate 0.5g; sodium chloride 5g; MgCl 2 1.7mM; MgSO 4 1.4mM; KCl, 0.47mM; CaCl 2 0.3mM; Dissolve in 900mL 50mM pH 7.5 Tris-HCl, dilute to 1000mL, aliquot and sterilize for 15min before use.
[0064] The optimal growth conditions for Bacillus sp.HJB17 are 37°C, pH 7.0. It was detected that the bacteria had phytase activity at 37°C (0...
Embodiment 2
[0065] Example 2 Cloning of Bacillus sp.HJB17 Phytase Encoding Gene phyH
[0066]Genomic DNA of Bacillus sp.HJB17 was extracted using a Bacterial Genome Extraction Kit (Tiangen Company), and the specific operation room instructions. According to the conserved sequence of BPP phytase gene, degenerate primers BPP-F and BPP-R were designed and synthesized: BPP-F: 5′-GACGCAGCCGAYGAYCCNGCNITNTGG-3′ and BPP-R: 5′-CAGGSCGCANRTCIACRTTRTT-3′. PCR amplification was performed using the total DNA of Bacillus sp.HJB 17 as a template. A fragment of about 180bp was obtained, which was recovered and connected to the pEASY-T3 vector for sequencing.
[0067] According to the nucleotide sequence obtained by sequencing, three TAIL-PCR specific primers were designed upstream and downstream: the design direction was the direction of the unknown region to be amplified, the position of sp2 was designed to be inside sp1, and sp3 was located inside sp2. The distance between every two primers is not s...
Embodiment 3
[0071] Embodiment 3 Preparation of recombinant phytase
[0072] Recombination and purification of PhyH:
[0073] The signal peptide-removed phytase-encoding gene phyH and the expression vector pET22b(+) were digested simultaneously (EcoRI+XhoI), connected overnight, and transformed into TransI-competent cells to obtain phytic acid containing Bacillus sp.HJB 17BPP The recombinant plasmid pET22(+)-phyH of the enzyme gene phyH was transformed into BL21(DE3) by the sequenced correct recombinant plasmid pET22(+)-phyH.
[0074] Take the BL21(DE3) strain containing the plasmid, inoculate it in 300mL LB culture medium, shake it at 220rpm at 37℃ for about 2h, then add 1mM IPTG and 1mM CaCl 2 , placed at 30° C. and 220 rpm for induction, and after about 8 hours, the intracellular and extracellular phytase activities were measured. The activity of phytase was detected in both intracellular and extracellular, and the intracellular was higher than the extracellular. Through nickel colum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com