4-Carboxyphenyl retinamide ethanolate and its preparation method and pharmaceutical composition
A technology of retinamide and carboxyphenyl, which is applied in the field of retinoid drug compounds, can solve problems affecting the quality control and practicability of drugs, unstable physical and chemical properties, and affecting industrial production, storage and transportation, and listing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Preparation and identification of 4-CPRE:
[0140] Add 95% ethanol (1 g: 2.5 ml) to the yellow 4-CPR solid, stir and react at room temperature for 30-60 minutes, and the solid turns from bright yellow to blood red completely. Filtrate, wash 3 times with 95% ethanol, avoid light, dry naturally to constant weight, the total yield is 62%, and the final product 4-CPRE in the form of blood red powder is obtained, which is a common crystal sample of type V (or directly prepared into other preferred crystal forms ); TLC (silica gel plate, petroleum ether / ethyl acetate / glacial acetic acid 1.5ml: 1ml: 1 drop development) Rf=0.62; other detections meet the pharmaceutical standards.
[0141] Melting point; 192~194℃
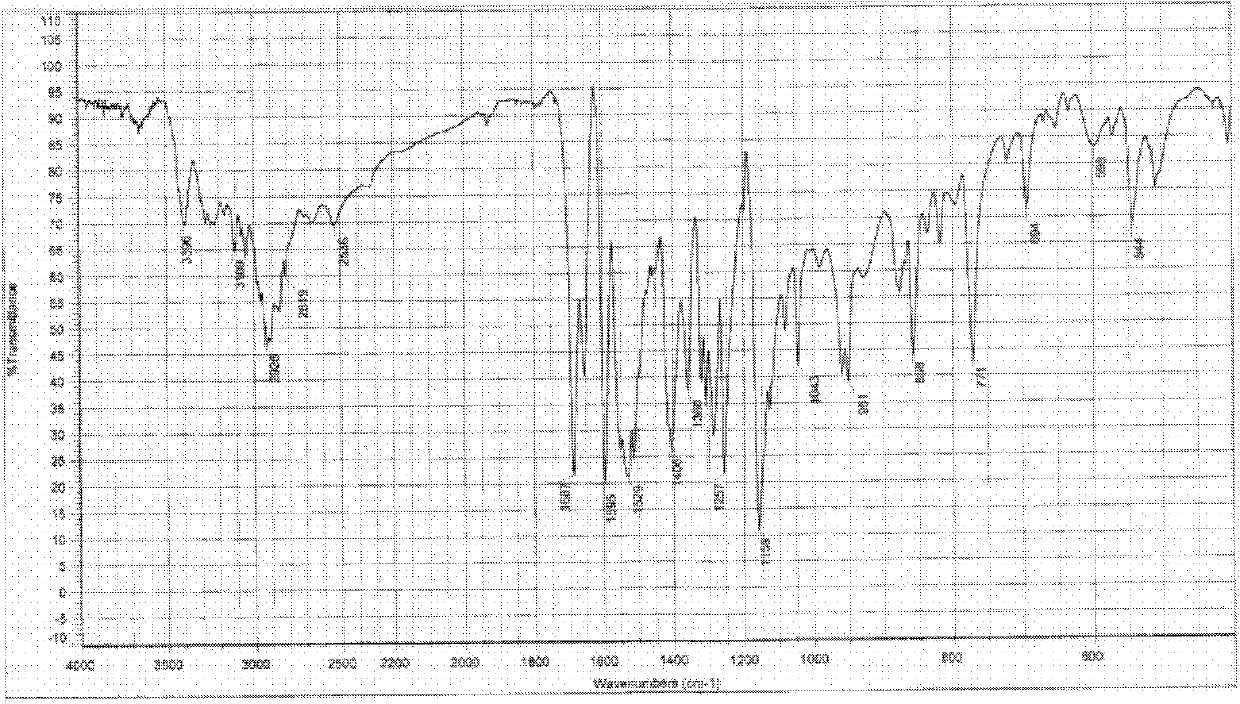
[0142] Infrared spectrum and data: KBr sheet, wavelength cm -1 , the data table is as follows:
[0143]3645, 3440, 3400, 3300-3050 (alcohol O-H stretching vibration, associative hydroxyl group), 2918, 2655, 2536, 2366, 1780, 1687 (aromatic acid carbonyl), 1665 (ami...
Embodiment 2
[0150] Preparation and Identification of Type IV Single Crystal of 4-CPRE:
[0151] Solvent method: In ethanol with low temperature (-18°C) and vigorous and rapid stirring, quickly add 4-CPRE samples dissolved in ethyl acetate (or ethylene glycol dimethyl ether) at room temperature, and continue to stir the mixture vigorously and rapidly until the crystallization is complete , filtered, washed, and dried to obtain type IV crystals, which should be stored away from light. The identification is as follows:
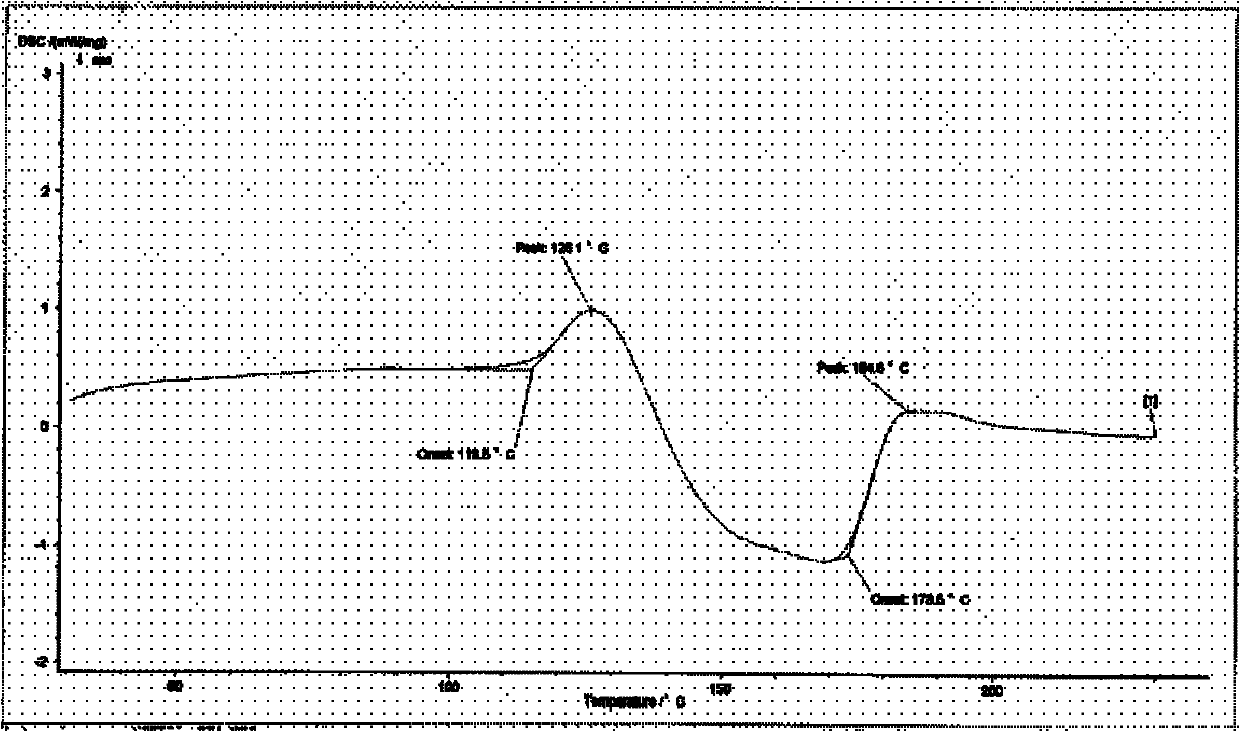
[0152] ①Differential thermal DSC spectrum:
[0153] Ti169.0°C, Tp181.8°C peaks and pseudopolymorph II Ti112.6°C, Tp126.1°C peaks, valley peak temperature 157.0°C, stable crystal form, proved to be a single crystal form, as shown in Figure 12.
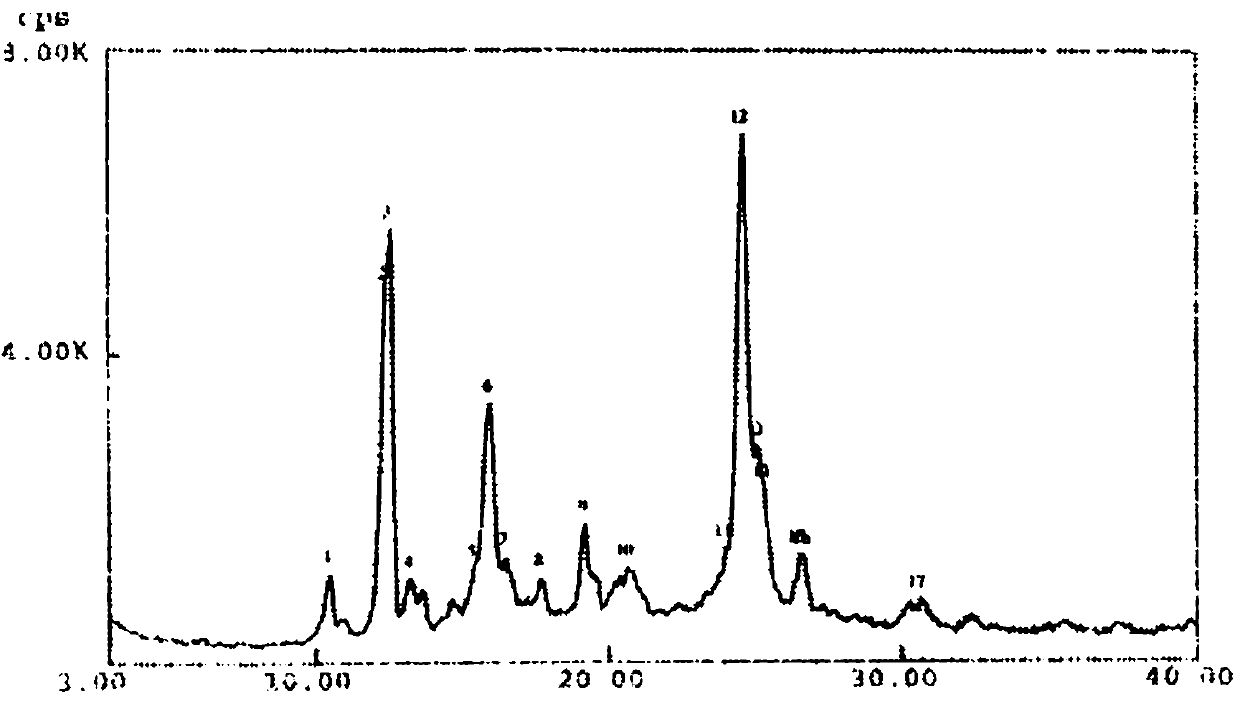
[0154] ②Crystal X-ray diffraction spectrum and data:
[0155] The results proved that the type IV single crystal form: (i) the nitrogen atom and amide oxygen atom of 4-CPR and the ethanol hydroxyl group were combined into 4-CPRE in...
Embodiment 3
[0161] 4-CPRE type IV single crystal energy conversion method:
[0162] ①Solid heating transformation method: the solid containing type III or type V is irradiated with infrared lamps and heated at 30-50°C for 12-6 hours, and the crystal is transformed into a single crystal type of type IV, see attached figure 2 , 3 . ②Refrigeration conversion method: When the solid containing type III or type V is placed at 4°C for about 730 days, 386 days or 275 days, it can be transformed into a single crystal form of type IV or an incompletely transformed polymorph, such as Figure 4 , 6 , 7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com