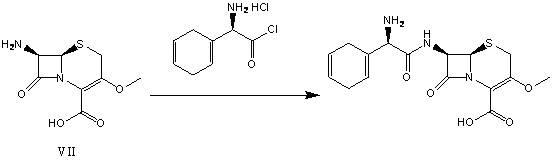
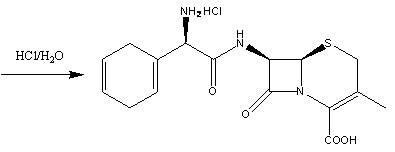
Synthetic intermediate compound of cephradine or cefroxadine and its preparation method and application
A technology of cephradine and its compound, which is applied in the direction of organic chemistry and bulk chemical production, can solve the problems of complex process, long post-processing operation cycle, difficulty in industrial production, etc., achieve high reaction yield, avoid isomerization phenomenon, easy The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
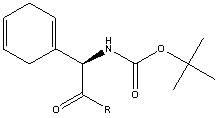
[0059] Preparation of D-2-(tert-butoxycarbonyl)amino-2-(1,4-cyclohexadiene)acetic acid (II)
[0060] Add 15.3g of D-2-amino-2-(1,4-cyclohexadiene) acetic acid, 8g of sodium hydroxide, 150g of water and 150g of THF into the reaction flask, cool to 0°C, add 23.6g of Boc 2 O, keep warm for 2 hours, rise to room temperature, react for 8 hours, recover THF, add ethyl acetate, adjust the pH of the aqueous phase to 3-4 with concentrated hydrochloric acid, separate layers, extract the aqueous phase with ethyl acetate, and combine the organic phases Finally, the organic layer was washed with saturated brine, concentrated and dried to obtain 25 g of crude D-2-(tert-butoxycarbonyl)amino-2-(1,4-cyclohexadiene)acetic acid.
[0061] Similarly, the catalyst can be replaced by potassium hydroxide, sodium carbonate, potassium carbonate, sodium bicarbonate or potassium bicarbonate as an option.
Embodiment 2
[0063] Preparation of D-2-(tert-butoxycarbonyl)amino-2-(1,4-cyclohexadiene)acetic acid (II)
[0064] Add 15.3g D-2-amino-2-(1,4-cyclohexadiene) acetic acid, 42g sodium bicarbonate, 306g water, 612g dioxane and 109g Boc 2 O, react at 0°C for 36 hours, recover THF, add DCM, adjust the pH of the aqueous phase to 3-4 with concentrated hydrochloric acid, separate layers, extract the aqueous phase with DCM, combine the organic phases, concentrate and dry to obtain D-2-( 20g of crude product of tert-butoxycarbonyl)amino-2-(1,4-cyclohexadiene)acetic acid.
[0065] Similarly, the catalyst can be replaced by sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate or potassium bicarbonate as an option.
Embodiment 3
[0067] Preparation of D-2-(tert-butoxycarbonyl)amino-2-(1,4-cyclohexadiene)acetic acid (II)
[0068] Add 15.3g of D-2-amino-2-(1,4-cyclohexadiene) acetic acid, 10g of potassium bicarbonate, 76.5g of water and 38.3g of THF into the reaction flask, cool to 0°C, add 21.8g of Boc 2 O, after 2 hours of heat preservation, rise to 35°C, react for 6 hours, recover THF, add ethyl acetate, adjust the pH of the aqueous phase to 3-4 with concentrated hydrochloric acid, separate layers, extract the aqueous phase with ethyl acetate, and combine the organic After phases, the organic layer was washed with saturated brine, concentrated and dried to obtain 22 g of crude product of D-2-(tert-butoxycarbonyl)amino-2-(1,4-cyclohexadiene)acetic acid.
[0069] Similarly, the catalyst can be replaced by sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate or sodium bicarbonate as an option.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com