Arylpyrazoline Luminescent Compounds Substituted by 5-Position Fused Aromatic Hydrocarbons
A light-emitting compound, arylpyrazoline technology, applied in the direction of light-emitting materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of little research, achieve high melting point, improve luminescence and two-photon fluorescence, thermal stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1.1 Synthesis of 3-(9-anthryl)-1-phenylpropyl-2-en-1-one (intermediate)
[0022] Add 1.20 g of acetophenone, 2.05 g of 9-anthral, and 20 ml of ethanol into a 100 ml three-necked flask, slowly add 6 ml of 3 mol / L NaOH aqueous solution dropwise under magnetic stirring at room temperature, react for 2 hours, and suction filter the resulting yellow solid with acetic acid Recrystallization yields yellow needle-like crystals. Yield: 85%, M.p.: 128-130°C.
[0023] 1.2 Synthesis of 1-(4-nitrophenyl)-3-phenyl-5-(9-anthracenyl)-2-pyrazoline (compound A)
[0024] Add 1.0g of intermediate A, 0.8g of p-nitrophenylhydrazine, 6ml of concentrated HCl, and 60ml of ethanol into a 100ml three-necked flask, react at 78°C for 6h under magnetic stirring, and then cool to room temperature, a yellow solid precipitates out. After suction filtration, the obtained solid was recrystallized from ethyl acetate to obtain yellow crystals. Yield: 74%, M.p.: 288-291°C, 1 HNMR (CDCl3): δ8.54 (d, J ...
Embodiment 2
[0029] 2.1 Synthesis of 3-(9-anthryl)-1-(4-nitrophenylpropyl)-2-en-1-one (intermediate)
[0030] Add 1.65g of 4-nitroacetophenone, 2.06g of 9-anthracene, and 20ml of ethanol into a 100ml three-neck flask, slowly add 6ml of 3mol / L NaOH aqueous solution under magnetic stirring at room temperature, react for 2 hours and then filter with suction to obtain a red solid Red needle-like crystals were obtained by recrystallization from ethyl acetate-acetic acid (v:v=1:1). Yield: 81%, M.p.: 158-160°C.
[0031] 2.2 Synthesis of 1,3-bis(4-nitrophenyl)-5-(9-anthracenyl)-2-pyrazoline (compound B)
[0032] Add 1.0g of intermediate B, 0.8g of p-nitrophenylhydrazine, 6ml of concentrated HCl, and 60ml of ethanol into a 100ml three-neck flask, react at 78°C for 6h under magnetic stirring, and cool to room temperature, an orange-red solid precipitates out. Suction filtration, and the solid was recrystallized from ethyl acetate to obtain orange-red crystals. Yield: 71%, M.p.: 309-310°C, 1 HNMR...
Embodiment 3
[0037] 3.1 Synthesis of 3-(4-nitrophenyl)-5-(9-anthracenyl)-2-pyrazoline (compound C)
[0038] Add 1.0g of the intermediate 3-(9-anthracenyl)-1-(4-nitrophenylpropyl)-2-en-1-one, 0.8g of hydrazine hydrate, 4ml of concentrated HCl, and 40ml of ethanol into a 100ml three-necked flask , reacted at 78°C for 6h under magnetic stirring, then cooled to room temperature, and a red solid precipitated out. After suction filtration, the obtained solid was recrystallized from ethanol to obtain red crystals. Yield: 70%, M.p.: 192-193°C.
[0039] 1 HNMR (CDCl 3 ): δ8.48(s, 1H), 8.36-8.38(m, 2H), 8.25-8.28(m, 2H), 8.03-8.06(m, 2H), 7.85-7.87(m, 2H), 7.48-7.52 (m, 4H), 6.55-6.60(t, 1H), 3.58-3.70(m, 2H)
[0040] 3.2 Optical performance test
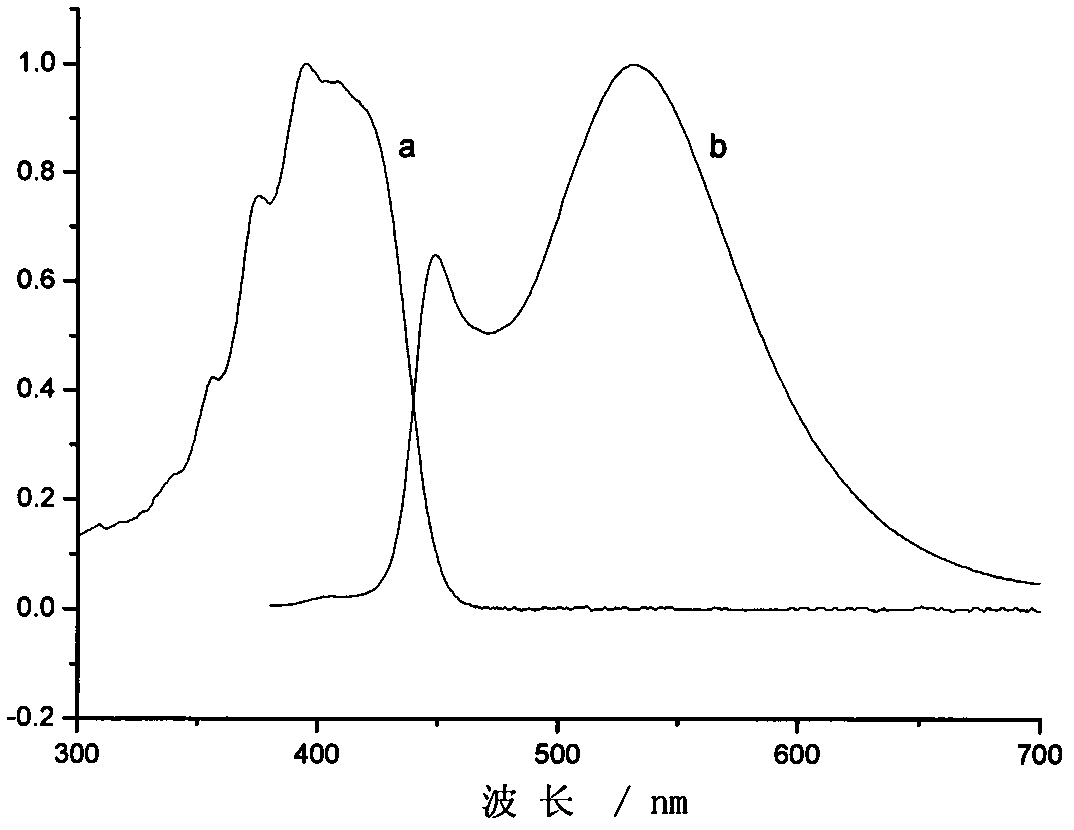
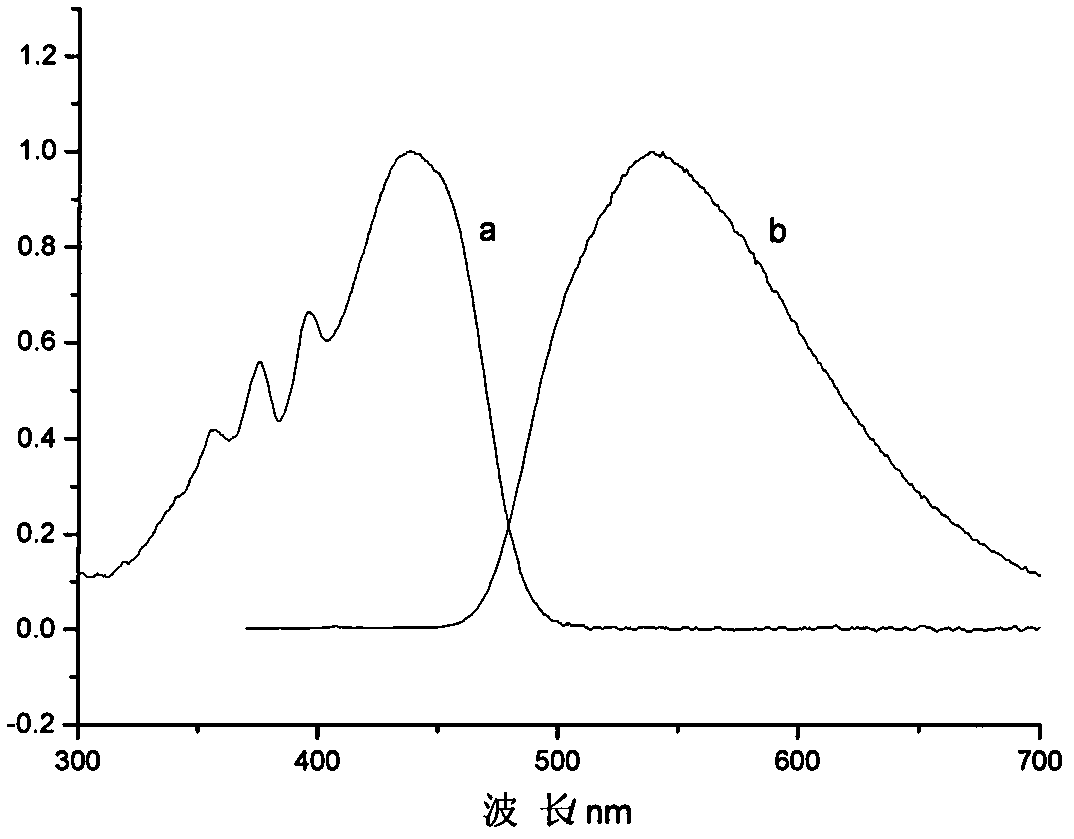
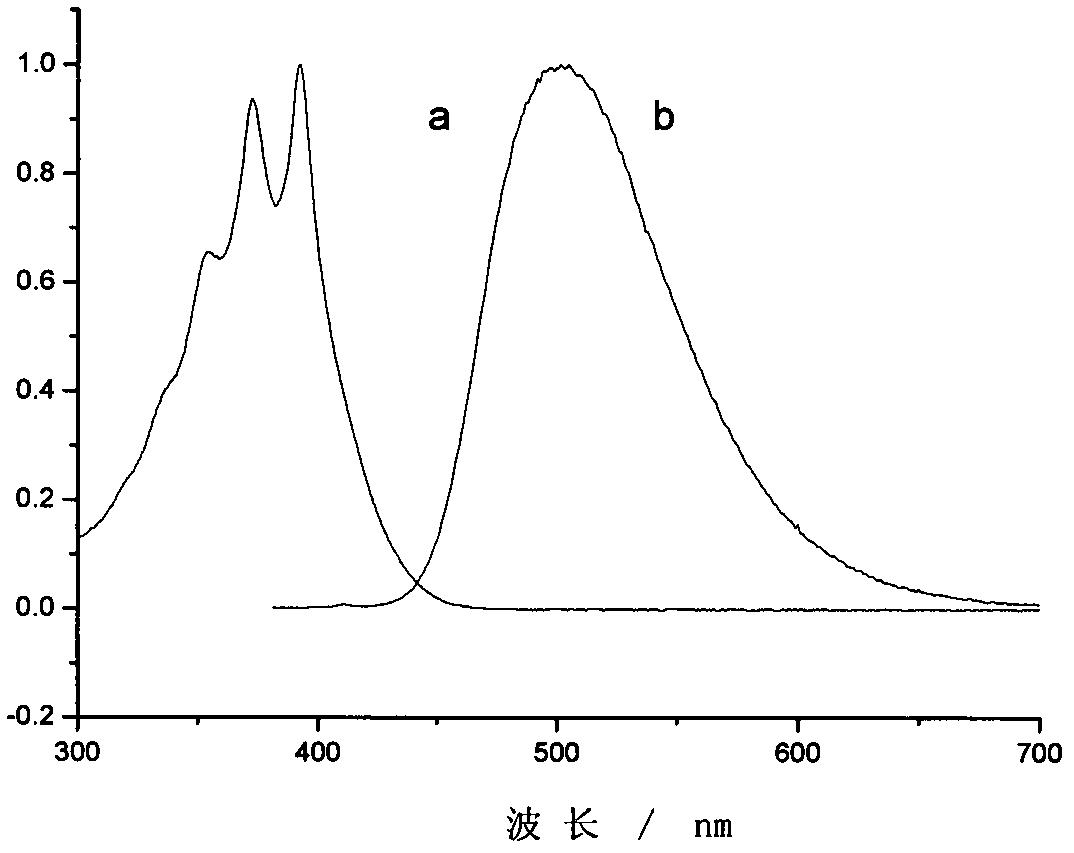
[0041]3-(4-nitrophenyl)-5-(9-anthracenyl)-2-pyrazoline was dissolved in toluene to prepare a dilute solution, and its ultraviolet absorption spectrum and fluorescence spectrum were measured. The absorption spectrum was measured on Shimadzu UV-3600 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com