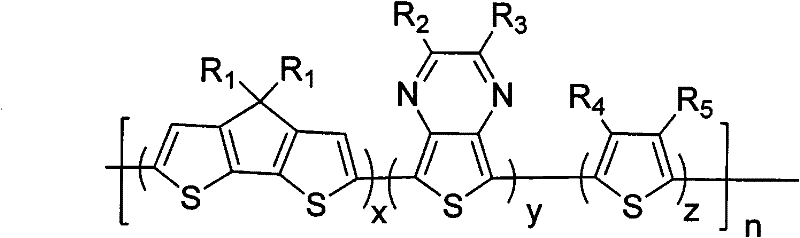
Cyclopentadiene-containing dithiophene-thienopyrazine-thiophene conjugated polymer and its preparation method and application
A technology containing cyclopentadiene dithiophene and cyclopentadiene dithiophene is applied in the field of organic compound synthesis, and can solve the problem that the red light region is not effectively utilized, the collection efficiency of the carrier electrode is low, and the carrier mobility is low. and other problems, to achieve the effects of good thermal stability and environmental stability, improved modifiability, and easy transfer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
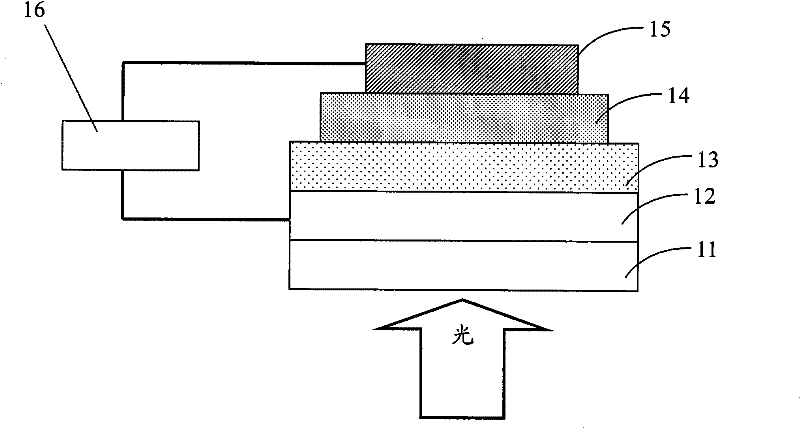
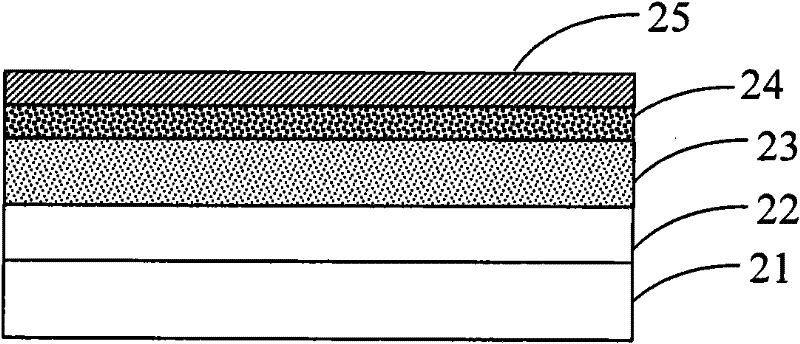
Image
Examples
preparation example Construction
[0042] This embodiment also provides a method for preparing the above-mentioned cyclopentadiene-containing dithiophene-thienopyrazine-thiophene conjugated polymer, including the following steps:
[0043] 1) Provide compounds A, B, C, and D represented by the following structural formula respectively,
[0044]
[0045] Among them, R 1 from C 1 ~C 20 the alkyl group; R 2 , R 3 selected from -H, C 1 ~C 20 Alkyl, C 1 ~C 20 Alkoxy groups, alkyl or alkoxy benzene ring groups, alkyl fluorene groups or alkyl carbazole groups; R 4 , R 5 selected from -H, C 1 ~C 20 Alkyl or C 1 ~C 20 alkoxy;
[0046] 2) In an oxygen-free environment and in the presence of a catalyzer and an organic solvent, select compounds A, B, C, and D to carry out a Stille coupling reaction to obtain a cyclopentadiene-containing dithiophene represented by the general formula (I)- Thienopyrazine-thiophene conjugated polymer, the Stille coupling chemical reaction formula of step 2) can be expressed a...
Embodiment 1
[0060] Preparation of cyclopentadiene(2,1-b:3,4-b')dithiophene-thiophene-2,3 bis(phenyl substituted)thieno[3,4-b]pyrazine conjugated polymers , its structural formula is as follows I 1 as shown,
[0061]
[0062] Its preparation steps are as follows:
[0063] 1) The preparation of compound 2,5-dibromo-4,5 dioctylthiophene, its chemical reaction formula is as follows:
[0064]
[0065] The specific preparation process is as follows: NBS (2.9g, 16.3mmol) was added to a DMF (50mL) solution containing 3,4-dioctylthiophene (2.1g, 6.8mmol), stirred at room temperature for 12 hours, and the reaction solution was poured into into saturated brine, followed by chloroform extraction, saturated salt book washing, rotary evaporation to remove the solvent to obtain a crude product, and the crude product was obtained by column chromatography (petroleum ether as eluent) to obtain an oily product with a yield of 76%. MS (EI) m / z of the product: 466 (M + ).
[0066]2) The preparation...
Embodiment 2
[0079] Cyclopentadiene (2,1-b:3,4-b') dithiophene-thiophene-2,3 bis(phenyl substituted)-thieno[3,4-b]pyrazine conjugated polymer Preparation, its structural formula is as follows I 2 as shown,
[0080]
[0081] Its preparation steps are as follows:
[0082] 1) According to the same preparation method and similar reaction conditions of step 1) in Example 1, the compound 2,5-dibromo-3,4-dioctyloxythiophene with the following structural formula was prepared,
[0083]
[0084] 2) According to the same preparation method and similar reaction conditions as step 2) in Example 1, 2-(4-n-butylphenyl) 3-(4-eicosyloxyphenyl) thieno with the following structural formula was prepared [3,4-b]pyrazine,
[0085]
[0086] 3) According to step 3) in Example 1, the same preparation method and similar reaction conditions were used to prepare 5,7-dibromo-2-(4-n-butylphenyl) 3-(4-eicosane) with the following structural formula Oxyphenyl)thieno[3,4-b]pyrazine,
[0087]
[0088] 4) A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com