Preparation method of hepatoma carcinoma cell-targeted polyamido-amine dendrimer support
A technology of polyamidoamine dendrimers and macromolecular carriers, which is applied in the direction of non-active ingredient medical preparations, pharmaceutical formulations, antineoplastic drugs, etc., can solve the problems of complex synthesis methods and no related reports, and achieve simple preparation methods , good biocompatibility, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) FITC modification of polyamidoamine dendrimers with fluorescein isothiocyanate:
[0036] In the 5th generation polyamidoamine dendrimer G5.NH containing 20 mg of amino-terminated 2 10 mL of anhydrous dimethyl sulfoxide (DMSO) solution containing 1.5 mg FITC was added dropwise to 10 mL of DMSO solution containing 1.5 mg FITC, and stirred with strong magnetic force at room temperature for 24 hours, and then the reaction product was successively dissolved in PBS with a cellulose dialysis membrane with a molecular weight cut-off of 10,000. Dialyze in buffer solution 4L×3 and ultrapure water 4L×3 for 3 days, and finally freeze-dry to obtain the product G5.NH 2 -FI.
[0037] (2) Partial acetylation modification of polyamidoamine dendrimers:
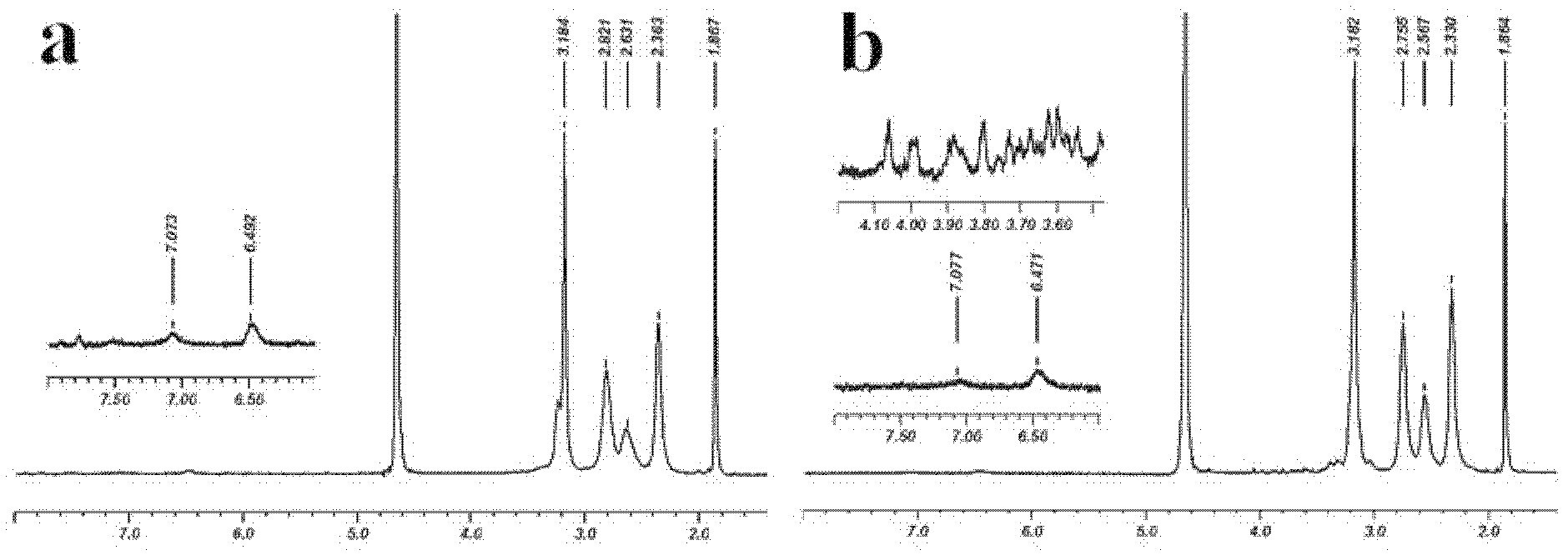
[0038] G5.NH 2-FI was dissolved in 10 mL of DMSO solution, mixed well with 9.6 μL of triethylamine, 5 mL of DMSO solution containing 7.1 mg of acetic anhydride was added dropwise, reacted for 24 hours under strong magnetic stirrin...
Embodiment 2
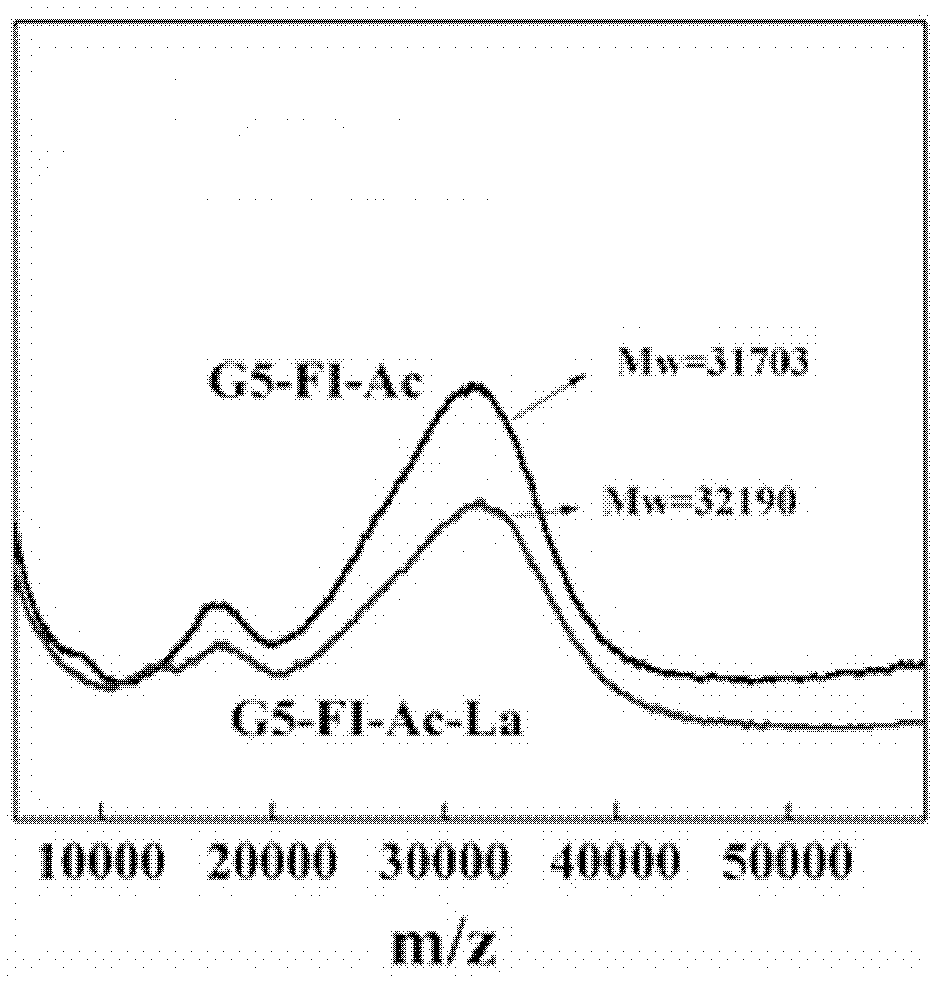
[0043] Synthesize two kinds of materials G5-FI-Ac-La and G5-FI-Ac according to the method of embodiment 1 and comparative example 1, carry out medium-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) test, the result is as follows attached figure 2 shown. The results showed that the molecular weight of the compound G5-FI-Ac-La modified with lactobionic acid was significantly higher than that of the compound G5-FI-Ac without modified lactobionic acid. Through calculation, it was found that there were 4 lactobionic acid molecules on the surface of G5-FI-Ac-La. Although there is a certain difference between this test result and the NMR result, considering that the test result of MALDI-TOF MS cannot reflect the average molecular weight of the material, it can only give a broad range of molecular weight distribution, so this result can still be proved very well Lactobionic acid has been successfully modified on the surface of dendrimers, and G5...
Embodiment 3
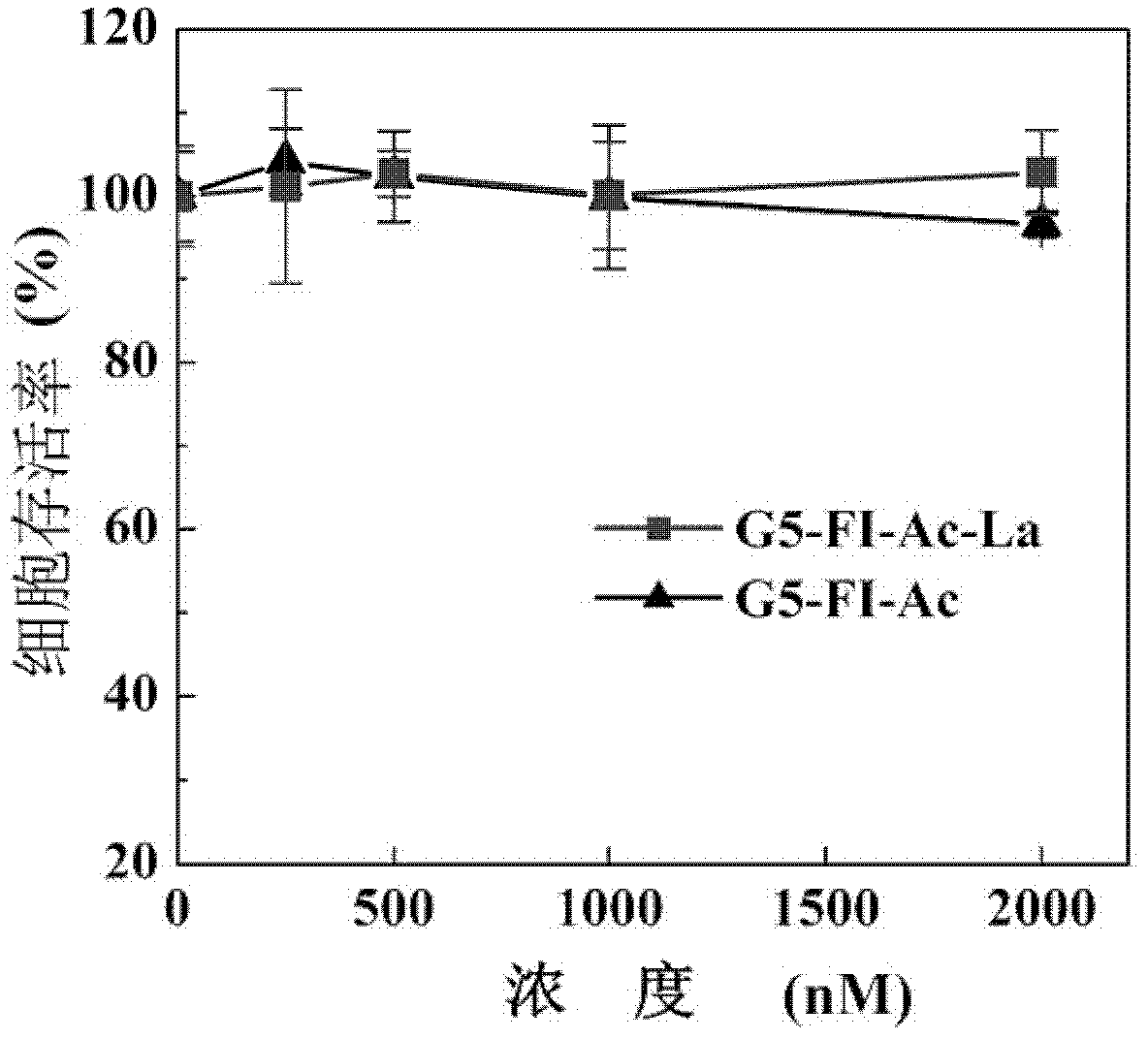
[0045] The cytotoxicity of the two materials G5-FI-Ac-La and G5-FI-Ac synthesized in Example 1 and Comparative Example 1 was tested using human liver cancer cell HepG2 as a model cell. After the model cells were cultured for 24 hours in the culture solution containing G5-FI-Ac-La and G5-FI-Ac at different concentrations, the survival of the model cells was tested by the MTT method, and the results are shown in the appendix image 3 . The result analysis shows that when the concentration range is 0-2000nM, after HepG2 cells are treated with G5-FI-Ac-La and G5-FI-Ac for 24 hours, the survival rate of the cells tested by MTT method is still higher than 95%. The carrier material synthesized by the invention has good biocompatibility and has no in vitro cytotoxicity within a certain concentration range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com