Cucoline derivative as well as salts, preparation method and application thereof
A technology of sinomenine and its derivatives, which is applied in the field of medicine, and can solve problems such as large medicinal doses, anaphylactic shock, and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
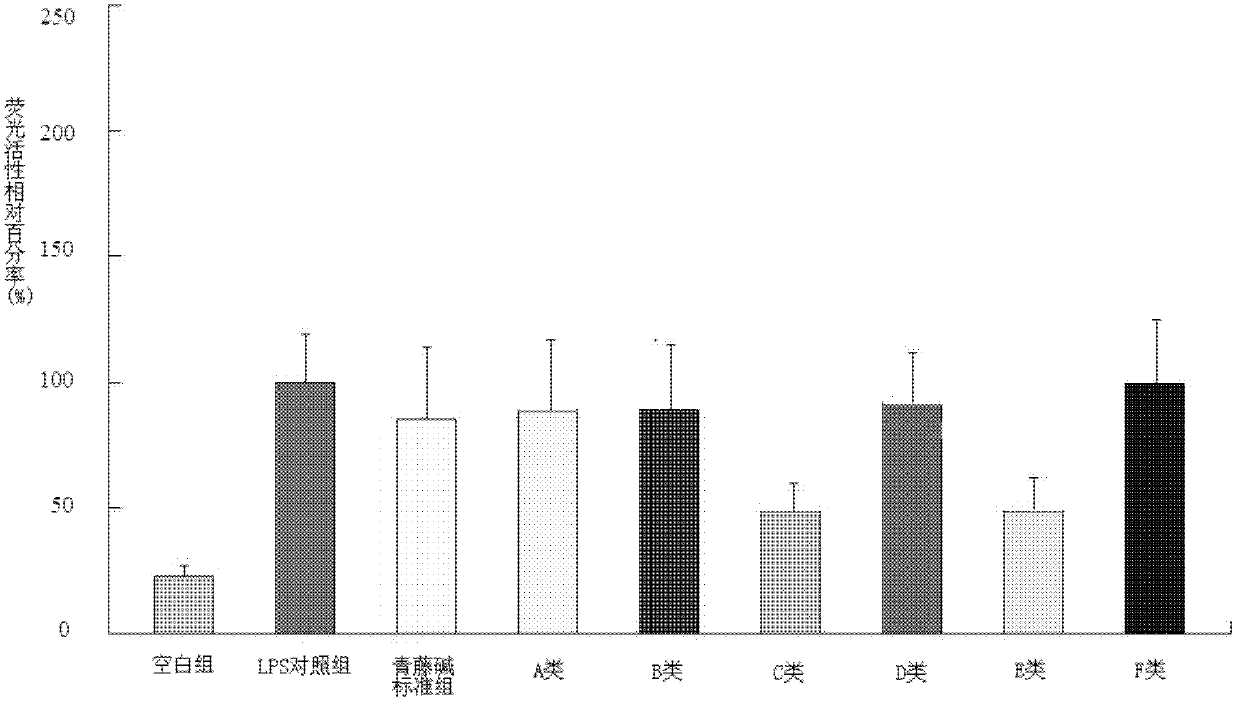
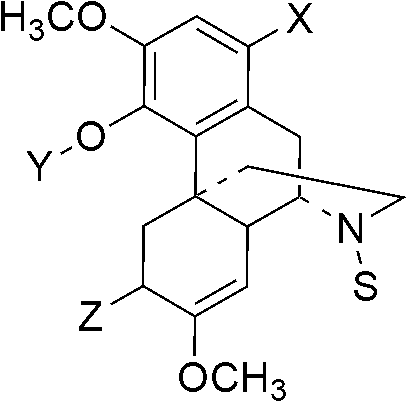
[0096] Example 1: Preparation of Class A sinomenine derivatives 1-bromo-substituted benzyl sinomenine ether
[0097] (1) Preparation of intermediate 1-bromosinomenine
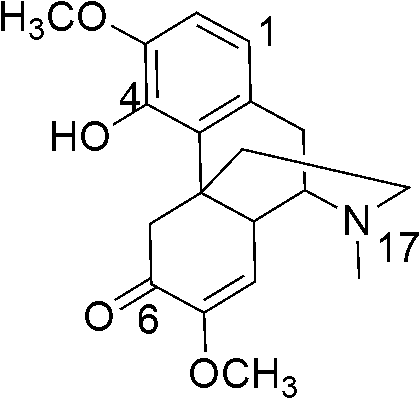
[0098] Mix 200mg (0.61mmol) of sinomenine, 130mg (0.73mmol) of N-bromosuccinimide (NBS), and 30ml of chloroform in an eggplant-shaped bottle, reflux and stir for 4 hours, concentrate the appropriate amount of the reaction solution, and stir The sample was applied to a silica gel column (eluent: dichloromethane) to obtain 227 mg of pure product with a yield of 91%. 1 H NMR (300MHz, CDCl 3 ): 6.93 (1H, s, H-2), 5.43-5.44 (2H, m, H-8), 2.47-4.36 (2H, dd, J=15.9Hz, H-5), 3.81 (3H, s, CH 3 O-3), 3.50 (3H, s, CH 3 O-7), 3.16-3.44 (2H, m, H-10), 2.99-3.05 (1H, m, H-9), 2.59-2.73 (2H, m, H-16), 2.55 (3H, s, H-17), 2.06-2.10 (1H, m, H-14), 1.97-1.99 (2H, m, H-15).
[0099] (2) Preparation of 1-bromo-4-methylbenzyl sinomenine ether
[0100] Dissolve 200mg (0.48mmol) of 1-bromosinomenine in 40ml of absolute ethanol...
Embodiment 2
[0102] Example 2: Preparation of Class B sinomenine derivatives 1-bromo-6(S)-hydroxyl-substituted benzyl sinomenine phenol ether
[0103] (1) Preparation of intermediate 6(S)-hydroxyl-sinomenine
[0104] Add 5 g (0.015 mol) of sinomenine to absolute ethanol, add 4.6 g (0.12 mol) of sodium borohydride in batches, reflux for 6 hours, evaporate ethanol under reduced pressure after cooling, and pour the oil into 40 ml of ice water , chloroform extraction (120ml×3), combined chloroform solution, concentrated to 100mL, added anhydrous sodium sulfate to dry for 6 hours, filtered, the filtrate was decompressed to remove chloroform, dissolved with ethyl acetate / petroleum ether, dropped a few drops of methanol, static Placed overnight, square crystals were precipitated, totaling 3.4 g, yield 67%. 1 H NMR (300MHz, CDCl 3 ): 6.55-6.71 (2H, dd, J=8.4Hz, H-1, H-2), 4.49 (1H, s, H-8), 3.04-4.18 (2H, m, H-5), 3.83 ( 3H, s, CH 3 O-3), 3.65-3.70 (1H, m, H-6), 3.45 (3H, s, CH 3 O-7), 2.75-2...
Embodiment 3
[0110] Example 3: Preparation of C-type sinomenine derivatives 1-substituted benzyl-1,2,3-triazole-4-methylene-sinomenine phenol ether
[0111] (1) Preparation of intermediate propynyl sinomenine phenol ether
[0112]Fully dissolve 3.29g (0.01mol) of sinomenine in a three-necked flask with 80ml of acetonitrile, add 1.12g (0.01mol) of potassium tert-butoxide, stir for 1 hour, then slowly drop in propyne bromide dissolved in 50ml of acetonitrile 3.57g (0.03mol), reacted for 2 hours. After the reaction was completed, it was concentrated to an appropriate amount, and the mixed sample was applied to a silica gel column (eluent: chloroform-methanol) to obtain 2.34 g of pure product with a yield of 64%. 1 H NMR (300MHz, CDCl 3 ): 6.72-6.79 (2H, dd, J=8.4Hz, H-1, H-2), 5.42-5.43 (1H, m, H-8), 4.74-4.89 (2H, m, H-18), 2.51-4.18 (2H, dd, J=15.6Hz, H-5), 3.79 (3H, s, CH 3 O-3), 3.46 (3H, s, CH 3 O-7), 3.40-3.48 (2H, m, H-10), 2.99-3.01 (2H, m, H-16), 2.84-2.87 (1H, m, H-9), 2.57 (3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com