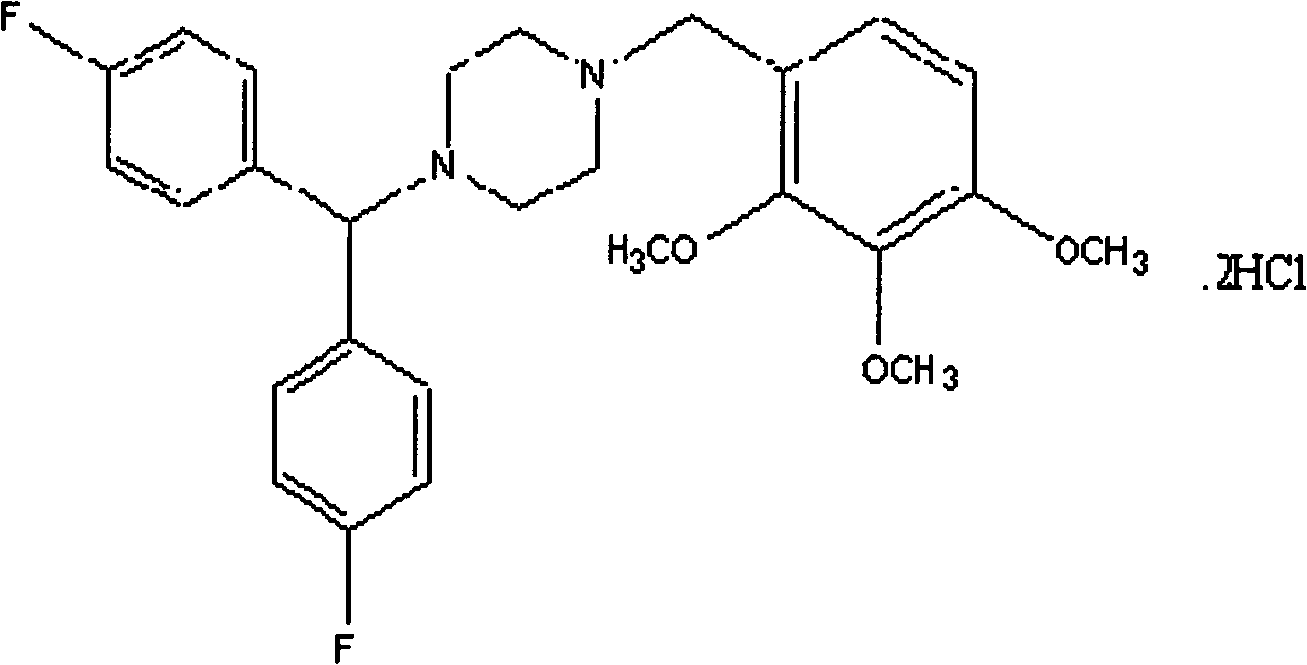
Lomerizine hydrochloride osmotic pump tablet and preparation method thereof
A technology of lomerizine hydrochloride and osmotic pump tablets, which is used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] prescription:
[0033]
[0034] Preparation method:
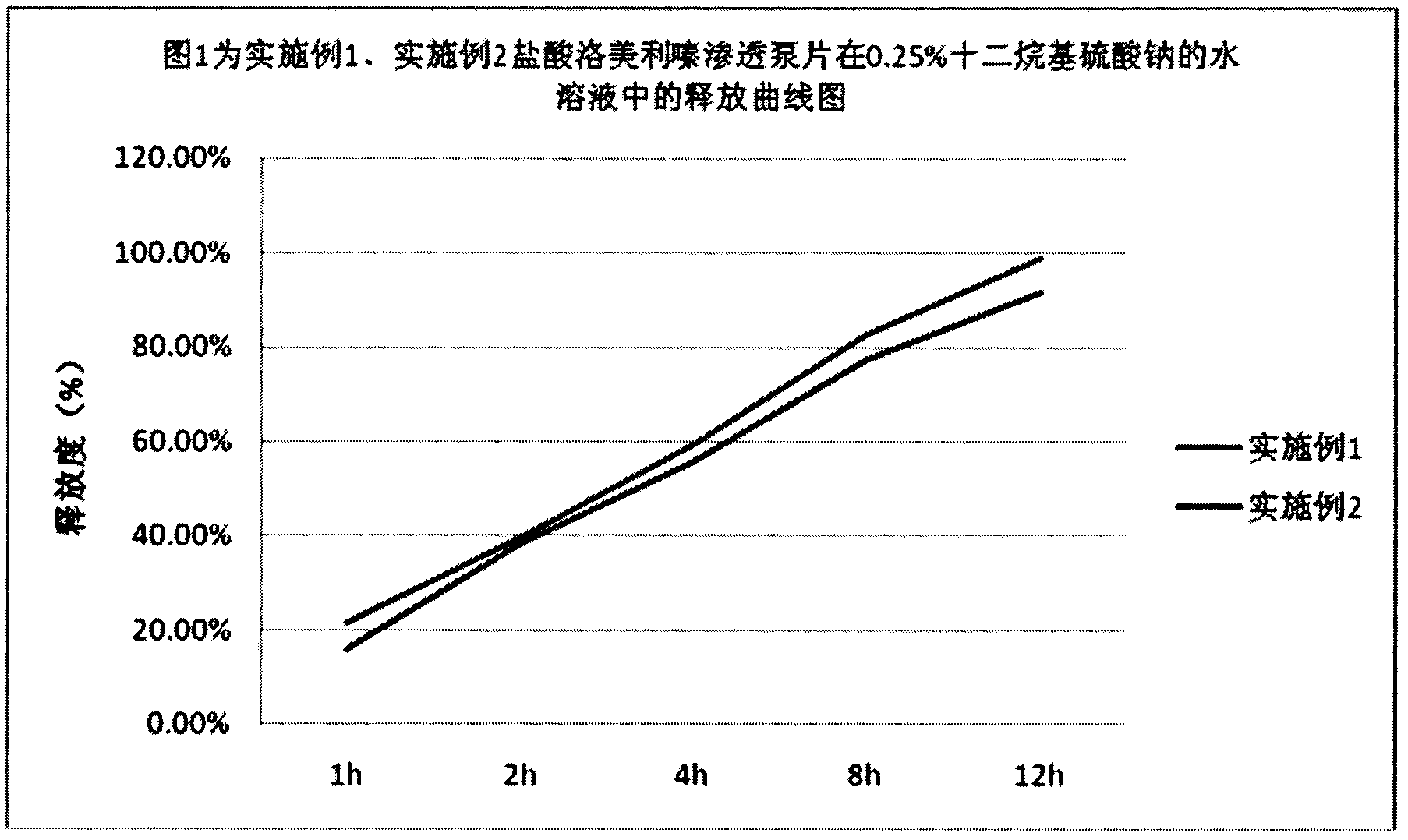
[0035] Grind lomerizine hydrochloride, meglumine, starch, mannitol, and povidone K30 respectively through a 100-mesh sieve, mix well, add water, and make a soft material, pass through a 24-mesh granulation, dry at 60°C for 2 hours, pass through 18-mesh granulation, adding magnesium stearate, mixing evenly, and pressing into tablet cores with a hardness of 2-8kg. Dissolve ethyl cellulose and triethyl citrate in acetone, mix well to obtain a coating solution, place the tablet core in a coating pan for coating, and cure at 50°C for 15 hours after coating. Prepare a small hole with a diameter of 0.2-0.8mm on one side of the chip core using a mechanical punching machine or a laser punching machine.
Embodiment 2
[0037] prescription:
[0038]
[0039] Preparation method:
[0040] Grind lomerizine hydrochloride, poloxamer, pregelatinized starch, lactose, and povidone K30 through a 100-mesh sieve, mix evenly, add water, and make a soft material, pass through a 24-mesh granulation, and dry at 60°C for 2 hour, cross 18 mesh granules, add magnesium stearate, mix uniformly, and compress into a tablet core with a hardness of 2-8kg. Dissolve methylcellulose and citric acid ester in acetone, mix evenly to obtain a coating solution, place the tablet core in a coating pan for coating, and cure at 50°C for 15 hours after coating. Prepare a small hole with a diameter of 0.2-0.8mm on one side of the chip core using a mechanical punching machine or a laser punching machine.
[0041] Study on the Release of Lomerizine Hydrochloride Osmotic Pump Tablets in 0.25% Sodium Lauryl Sulfate Aqueous Solution
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com