Chlorochromone acylhydrazone derivative based on rhodamine B as parent and application of derivative as fluorescence probe
A chromone acylhydrazone, fluorescent probe technology, applied in biological testing, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of long copper ion time, shorten detection time, etc., achieve high sensitivity, speed up detection, penetration Sex-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
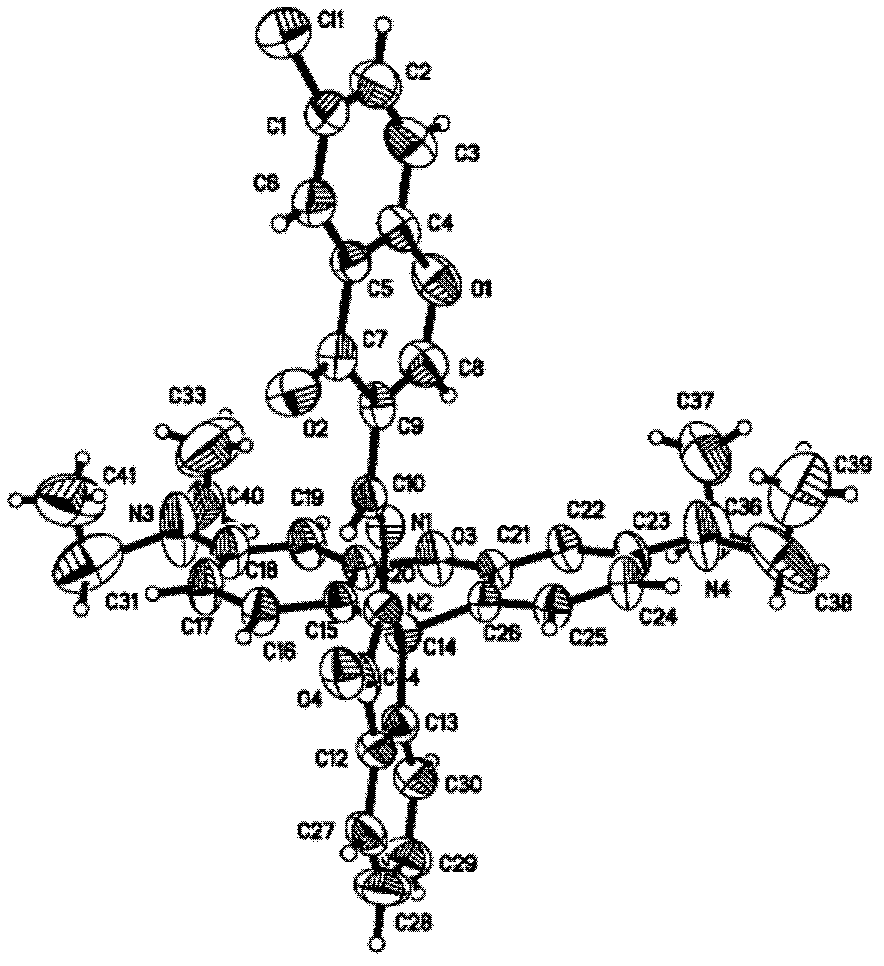
[0023] The reaction process of the chlorochromone acylhydrazone derivatives of rhodamine B synthetic rhodamine B is shown in the following formula:
[0024]
[0025] The specific preparation method is as follows:
[0026] Add 1g Rhodamine B (1) (2.1mmol) and 2.7g 80wt% hydrazine hydrate (43mmol) into 12mL of absolute ethanol, heat to reflux, and continue the reaction for about three hours. After the reaction is complete, reduce the temperature and concentrate under reduced pressure to remove the solvent. Add 40mL of water and 40mL of ethyl acetate to the mixture, shake and extract, continue to extract the water phase twice with ethyl acetate, combine the organic phases, dry over anhydrous magnesium sulfate, filter with suction, and concentrate under reduced pressure to remove the solvent to obtain khaki-yellow Rhodamine B Hydrazide (2) 0.95g, melting point: 166-170°C, yield: 97%.
[0027] H NMR spectrum determination: 1 H NMR (CDCl 3 , 400MHz), δ (ppm): 1.17 (t, 12H, NCH...
Embodiment 2
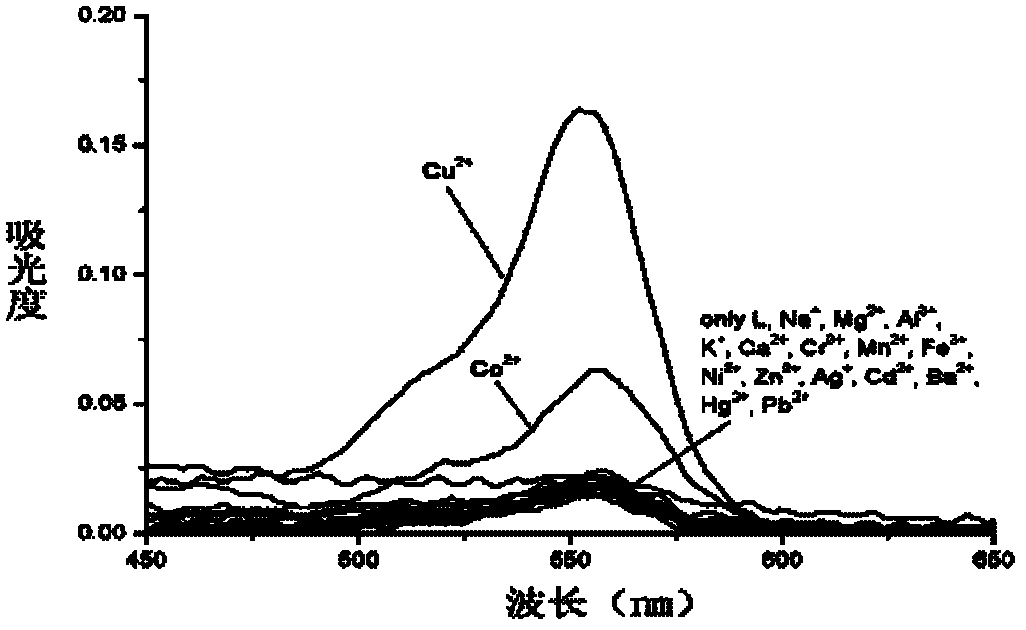
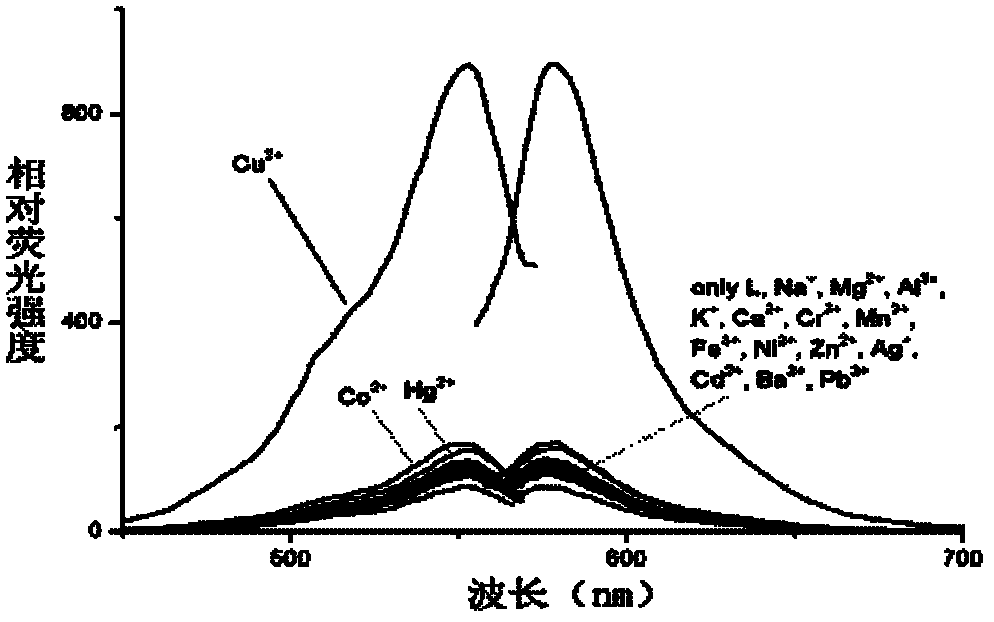
[0033] Add 10 equiv. Na + , Mg 2+ , Al 3+ , K + , Ca 2+ , Cr 3+ , Mn 2+ , Fe 3+ ,Co 2+ , Ni 2+ , Zn 2+ , Ag + , Cd 2+ , Ba 2+ , Pb 2+ , Hg 2+ and Cu 2+ The ionic water solution was tested by UV-Vis spectrophotometry and fluorescence spectrophotometry respectively.
[0034] The results show that the chlorochromone acylhydrazone derivatives of rhodamine B show better selectivity to divalent copper ions, and the contrast before and after adding copper ions shows a stronger fluorescence enhancement effect (see image 3 ).
Embodiment 3
[0036] Intracellular fluorescence imaging test:
[0037] A: use copper chloride, B: use copper nitrate. Control group 1 in each group of A and B: HeLa cells added with 10 μM rhodamine B chlorochromone acylhydrazone derivatives were cultured at 37°C for one hour; control group 2: HeLa cells added with 50 μM copper ions in the culture medium Incubate at 37°C for two hours, and then treat with the chromone acylhydrazone derivative of rhodamine B under the same conditions as the control group. Fluorescence imaging showed that the chlorochromone acylhydrazone derivatives of rhodamine B penetrated into cells well. Control group 1 showed no fluorescence, and control group 2 showed strong fluorescence in cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com