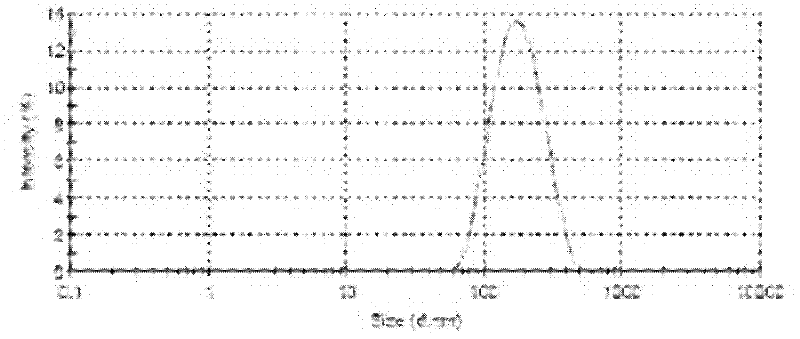
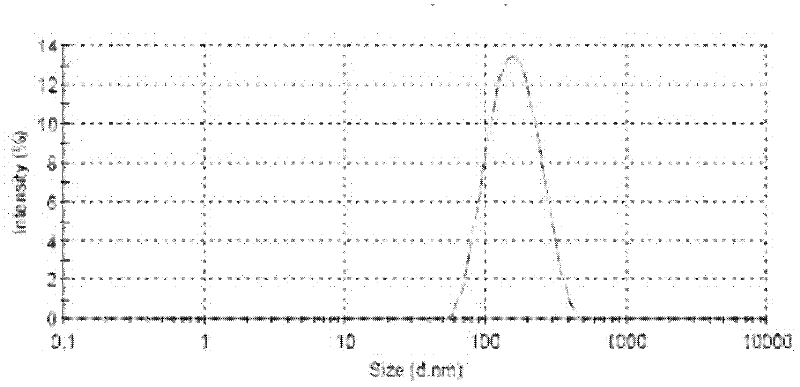
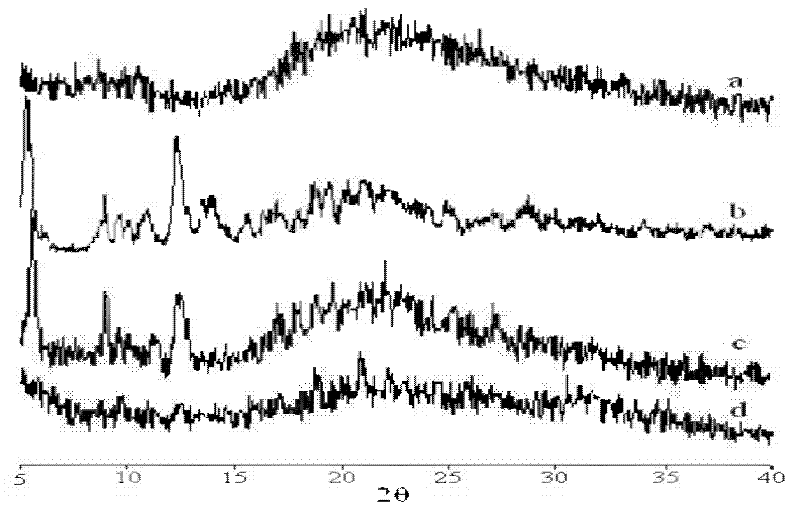
Protein nanometer granules wrapped with taxane medicaments and preparation method for nanometer granules
A taxane and nanoparticle technology, which is applied in the field of protein nanoparticle preparation, can solve the problems of loss of curative effect, unfavorable tumor targeting, easy failure of drugs, etc., and achieves improved convenience and safety of medication, good in vitro and in vivo Effects of stability, good pharmacodynamic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Preparation of paclitaxel albumin nanoparticles by melting method
[0081] 500 mg paclitaxel was dissolved in 9.0 ml ethanol. After 1200 mg of polyethylene glycol is completely melted by heating in an oil bath at 60°C, the paclitaxel solution is added to it, magnetically stirred until it is completely mixed and uniform, and after the ethanol is removed by rotary evaporation, it is rapidly cooled under vigorous stirring. After drying under vacuum overnight, the solid dispersion was added to 85 ml of human serum albumin aqueous solution (4.5% w / v, g / ml, the same below). The mixture was premixed by a high-speed disperser (XHF-1, Shanghai Jinda Biochemical Instrument Factory) for 1 minute to form a crude milk, and then transferred to a high-pressure homogenizer (EmulsiFlex-05, Avestin Company, Canada). High pressure homogenization at 5000-30,000 lb / in 2 Under the conditions of, the emulsion is repeated at least 5 times to obtain a protein nanoparticle suspension, an...
Embodiment 2
[0083] Example 2 Preparation of paclitaxel albumin nanoparticles by melt-solvent volatilization method
[0084] 300 mg paclitaxel was dissolved in 6.0 ml ethanol. 900 mg of polyethylene glycol was dissolved in 1.5 ml of absolute ethanol. After heating in an oil bath at 45°C, the paclitaxel solution was added to it, and the paclitaxel solution was added to it. Magnetically stirred until it was completely mixed. After the ethanol was removed by rotary evaporation, it was quickly stirred under vigorous stirring. cool down. After drying under vacuum overnight, the solid dispersion was added to 65 ml of human serum albumin aqueous solution (4.5% w / v). The mixture was premixed by a high-speed disperser (XHF-1, Shanghai Jinda Biochemical Instrument Factory) for 1 minute to form a crude milk, and then transferred to a high-pressure homogenizer (EmulsiFlex-05, Avestin Company, Canada). Emulsify at 5000-30,000 lb / inch 2 The emulsion was repeated at least 6 times to obtain a protein nanop...
Embodiment 3
[0086] Example 3 Preparation of Aseptic Filterable Paclitaxel Albumin Nanoparticles Less than 200 nm
[0087] 500 mg paclitaxel was dissolved in 9.0 ml ethanol. 800 mg polyethylene glycol was dissolved in 1.5 ml of absolute ethanol. After heating in an oil bath at 45°C, the paclitaxel solution was added to it, and the paclitaxel solution was added to it, magnetically stirred until it was completely mixed. After the ethanol was removed by rotary evaporation, it was quickly stirred under vigorous stirring. cool down. After drying under vacuum overnight, the solid dispersion was added to 97 ml of human serum albumin aqueous solution (4.5% w / v). The mixture was premixed by a high-speed disperser (XHF-1, Shanghai Jinda Biochemical Instrument Factory) for 1 minute to form a crude milk, and then transferred to a high-pressure homogenizer (EmulsiFlex-05, Avestin Company, Canada). Emulsify at 10000-40,000 lb / inch 2 The emulsion was repeated at least 6 times to obtain a protein nanoparti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com