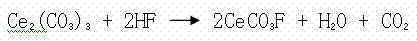Defluorination method for zinc sulphate solution
A zinc sulfate solution and removal technology, which is applied in the direction of zinc sulfate, chemical instruments and methods, and rare earth metal compounds, can solve the problems of large amount of fluorine absorbing and desorbing agent, limited ability to absorb fluorine, and unsatisfactory removal effect. High recovery rate and quality, strong pertinence, short and easy fluoride removal process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
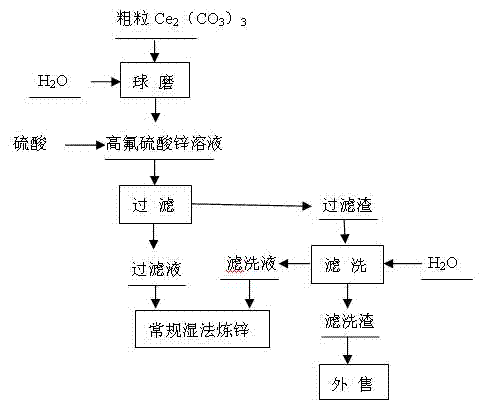
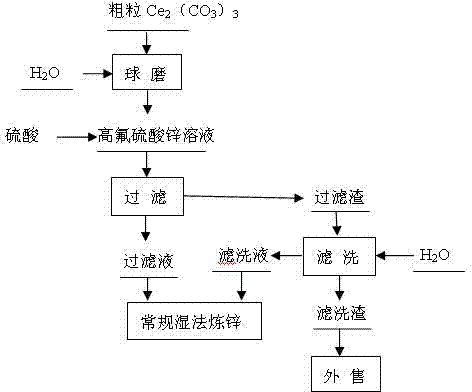
[0024] A method for removing fluorine in zinc sulfate solution, comprising the steps in the following order: A. ball milling process: adopt ceramic ball mill, add coarse Ce in the ball mill 2 (CO 3 ) 3 , add water to the ball mill, Ce in the ball mill 2 (CO 3 ) 3 The mass ratio of Ce to water is 1:1, and the Ce 2 (CO 3 ) 3 Wet milling, ball milling to make Ce 2 (CO 3 ) 3 Particle size 2 (CO 3 ) 3 Form slurry, slurry is used for precipitation defluorination process; B. precipitation defluorination: the slurry 243Kg (dry weight) of step A is added to 60 m 3 In the zinc sulfate solution with fluorine content of 0.311g / L and pH value of 5.0, the added Ce 2 (CO 3 ) 3 The mass ratio of fluorine to the zinc sulfate solution is 13:1, add sulfuric acid, the sulfuric acid is industrial sulfuric acid with a mass percentage concentration > 92%, adjust the pH value of the zinc sulfate solution to 3.0, control the temperature of the zinc sulfate solution to 60 ° C, pass Ce 2...
Embodiment 2
[0026] A method for removing fluorine in zinc sulfate solution, comprising the steps in the following order: A. ball milling process: adopt ceramic ball mill, add coarse Ce in the ball mill 2 (CO 3 ) 3 , add water to the ball mill, Ce in the ball mill 2 (CO 3 ) 3 The mass ratio to water is 1.5:1, the Ce 2 (CO 3 ) 3 Wet milling, ball milling to make Ce 2 (CO 3 ) 3 Particle size 2 (CO 3 ) 3 Form the slurry, which is used for precipitation defluorination process; B. precipitation defluorination: the slurry 361Kg (dry weight) of step A is added to 60 m 3 In the zinc sulfate solution with fluorine content of 0.43g / L and pH value of 5.2, the added Ce 2 (CO 3 ) 3 The mass ratio of fluorine to the zinc sulfate solution is 14:1, add sulfuric acid, the sulfuric acid is industrial sulfuric acid with a mass percentage concentration > 92%, adjust the pH value of the zinc sulfate solution to 4.0, control the temperature of the zinc sulfate solution to 70 ° C, pass Ce 2 (CO ...
Embodiment 3
[0028] A method for removing fluorine in zinc sulfate solution, comprising the steps in the following order: A. ball milling process: adopt ceramic ball mill, add coarse Ce in the ball mill 2 (CO 3 ) 3 , add water to the ball mill, Ce in the ball mill 2 (CO 3 ) 3 The mass ratio of Ce to water is 2:1, and the Ce 2 (CO 3 ) 3 Wet milling, ball milling to make Ce 2 (CO 3 ) 3 Particle size 2 (CO 3 ) 3 Form slurry, slurry is used for precipitation defluorination process; B. precipitation defluorination: the slurry 434Kg (dry weight) of step A is added to 60 m 3 In the zinc sulfate solution with fluorine content of 0.482g / L and pH value of 5.4, the added Ce 2 (CO 3 ) 3 The mass ratio of fluorine to the zinc sulfate solution is 15:1, add sulfuric acid, the sulfuric acid is industrial sulfuric acid with a mass percentage concentration > 92%, adjust the pH value of the zinc sulfate solution to 4.5, and control the temperature of the zinc sulfate solution to 80°C. Ce 2 (C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com