Angiotensin converting enzyme inhibitory peptide milky white peptide C1 and application thereof
An angiotensin and converting enzyme technology, applied in the field of pharmacology, can solve the problems of reduced activity and loss of analog activity, and achieve the effects of long half-life, easy operation and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
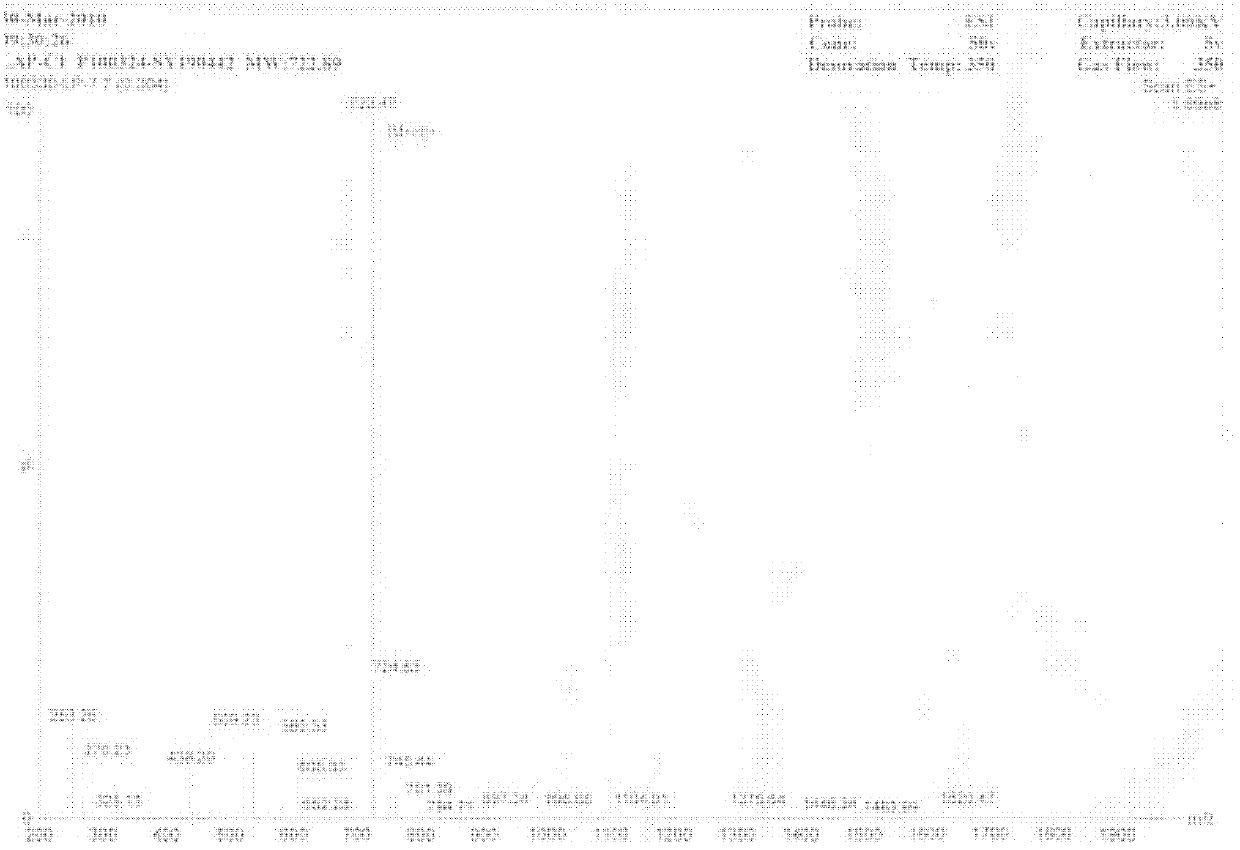
[0020] Synthesis of angiotensin-converting enzyme inhibitory peptide LAP-C1: angiotensin-converting enzyme inhibitory peptide LAP-C1 was synthesized by a solid-phase synthesizer, identified by a mass spectrometer, see the simple diagram figure 1 .
[0021] (1) Synthetic raw materials and related reagents:
[0022] 1).FMOC-Tyr-OH (FMOC-Tyr-OH), FMOC-Thr-OH, FMOC-Asn-OH, FMOC-Pro-OH, FMOC-Lys-OH, FMOC-Leu-OH, FMOC- Phe-OH, FOMC-Asp-OH, FMOC-Met-OH, FMOC-Arg-OH, etc. (Gill Biochemical (Shanghai) Co., Ltd.)
[0023] 2). Benzotriazole-N, N, N', N'-tetramethyluronium hexafluorophosphate (HBTU) (Gill Biochemical (Shanghai) Co., Ltd.)
[0024] 3).NMM (Sinopharm Group Shanghai Chemical Reagent Company)
[0025] 4). Dimethylformamide (DMF) (Dikma company)
[0026] 5).DCM (Dikma Company)
[0027] 6). Acetonitrile (Fisher company)
[0028] 7).FMOC-Tyr-Wang-Resin (FMOC-Tyr-Wang-Resin) (Gill Biochemical (Shanghai) Co., Ltd.)
[0029] 8). Piperidine (Sinopharm Shanghai Chemical Reagen...
Embodiment 2
[0049] IC50 value of LAP-C1 inhibiting ACE activity in vitro
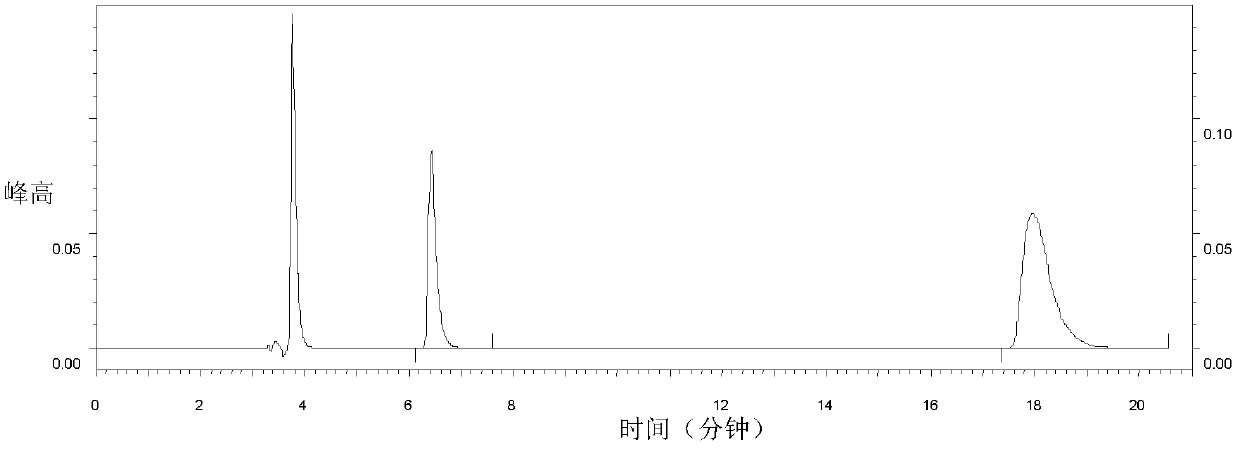
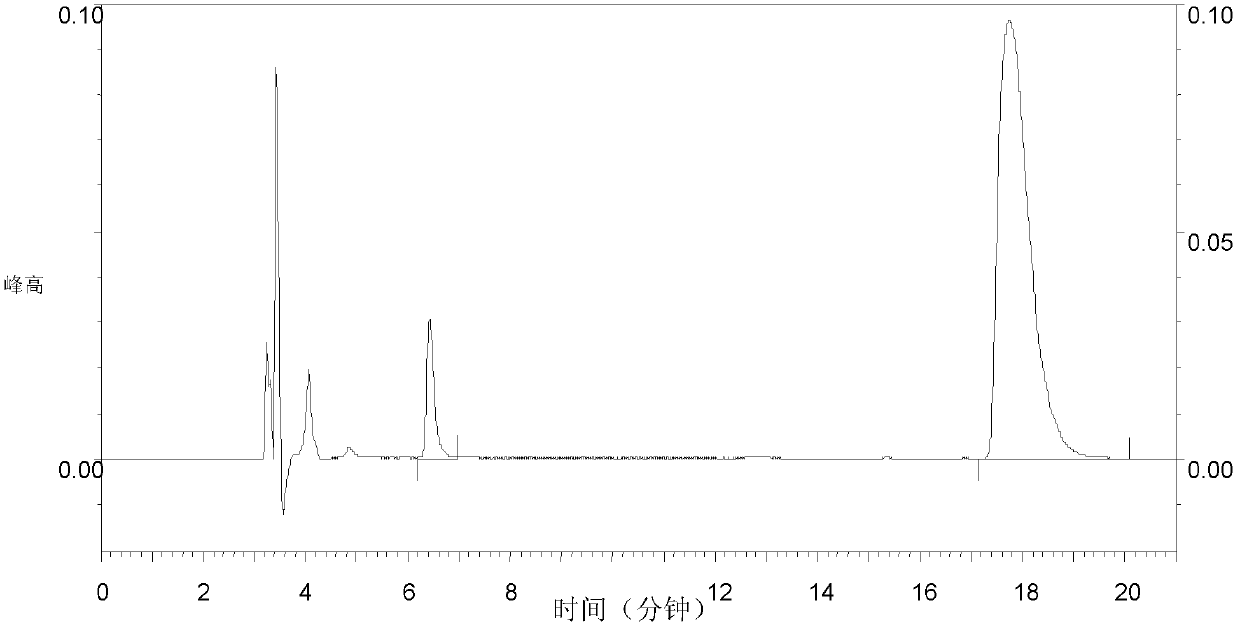
[0050]The activity of LAP-C1 in inhibiting angiotensin-converting enzyme was determined by direct injection and isocratic elution using a high-pressure liquid chromatography analyzer. Results In the analysis of the control group a (LAP-C1 was replaced by buffer), the concentration (1.523mmol / L) of the substrate hippuryl histidyl leucine (SIGMA H1635) (HHL) before the injection was higher than that of the HHL standard (0.29mmol / L) nearly 5 times higher, figure 2 It is Hip 0.112mmol / L and HHL 0.29mmol / L standard spectrum. image 3 Control group a: 50ul ACE + 10ul buffer + 200ul HHL (1.523mmol / L) + 250HCL, angiotensin converting enzyme activity map. image 3 The peak area of the peak at 17.743 minutes and figure 2 about 1.8 times higher than figure 2 The peak area of the peak at 6.43 minutes and image 3 It is about 2.9 times higher than the comprehensive figure 2 , image 3 The results shown suggest th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com