Application and preparation method of tripterine
A technology for the use of tripterygium, which is applied in the field of tripterygium, can solve the problems of cumbersome extraction process, high cost, high equipment investment, etc., and achieve the effect of high product purity, high raw material utilization rate and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
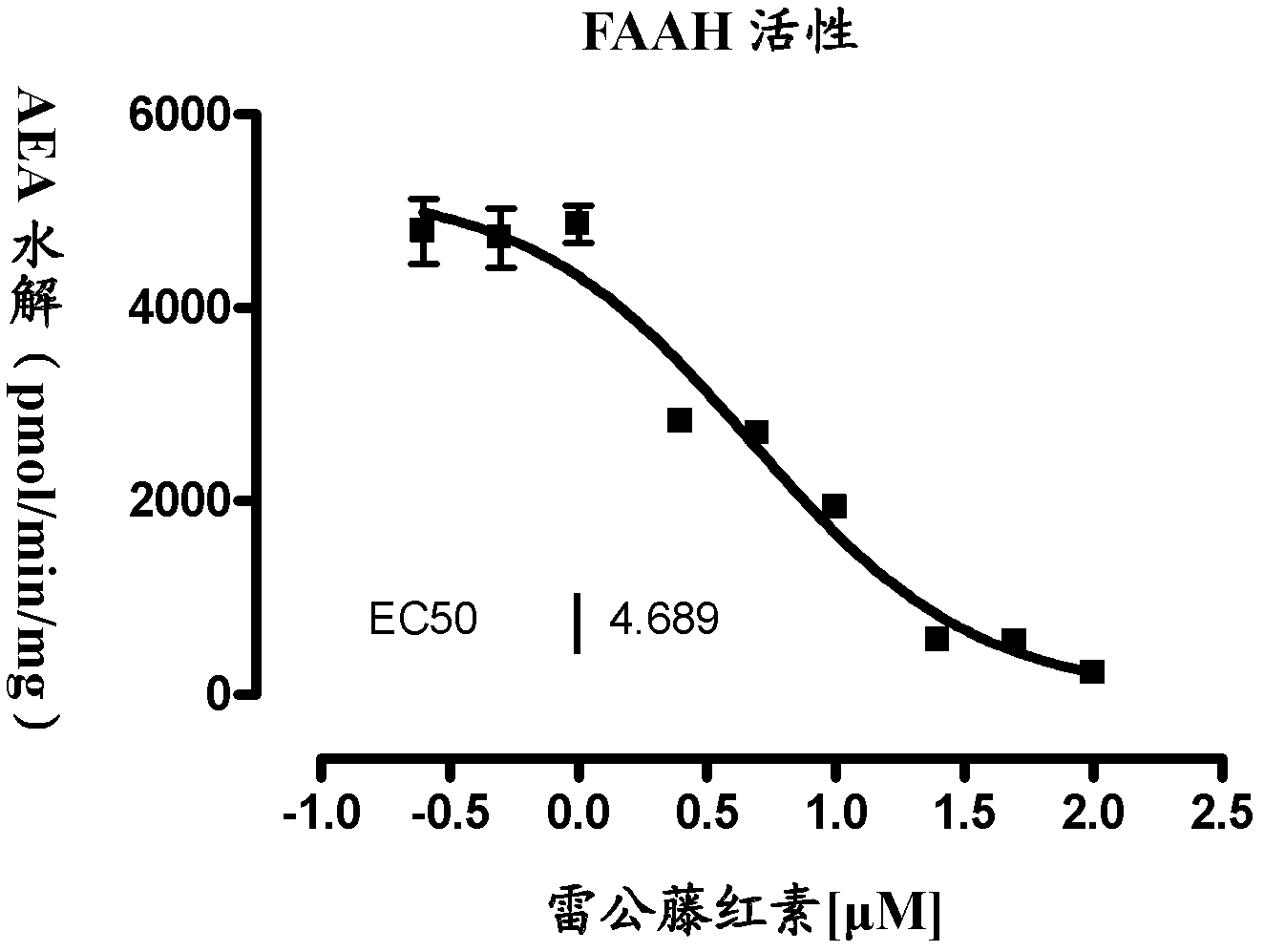
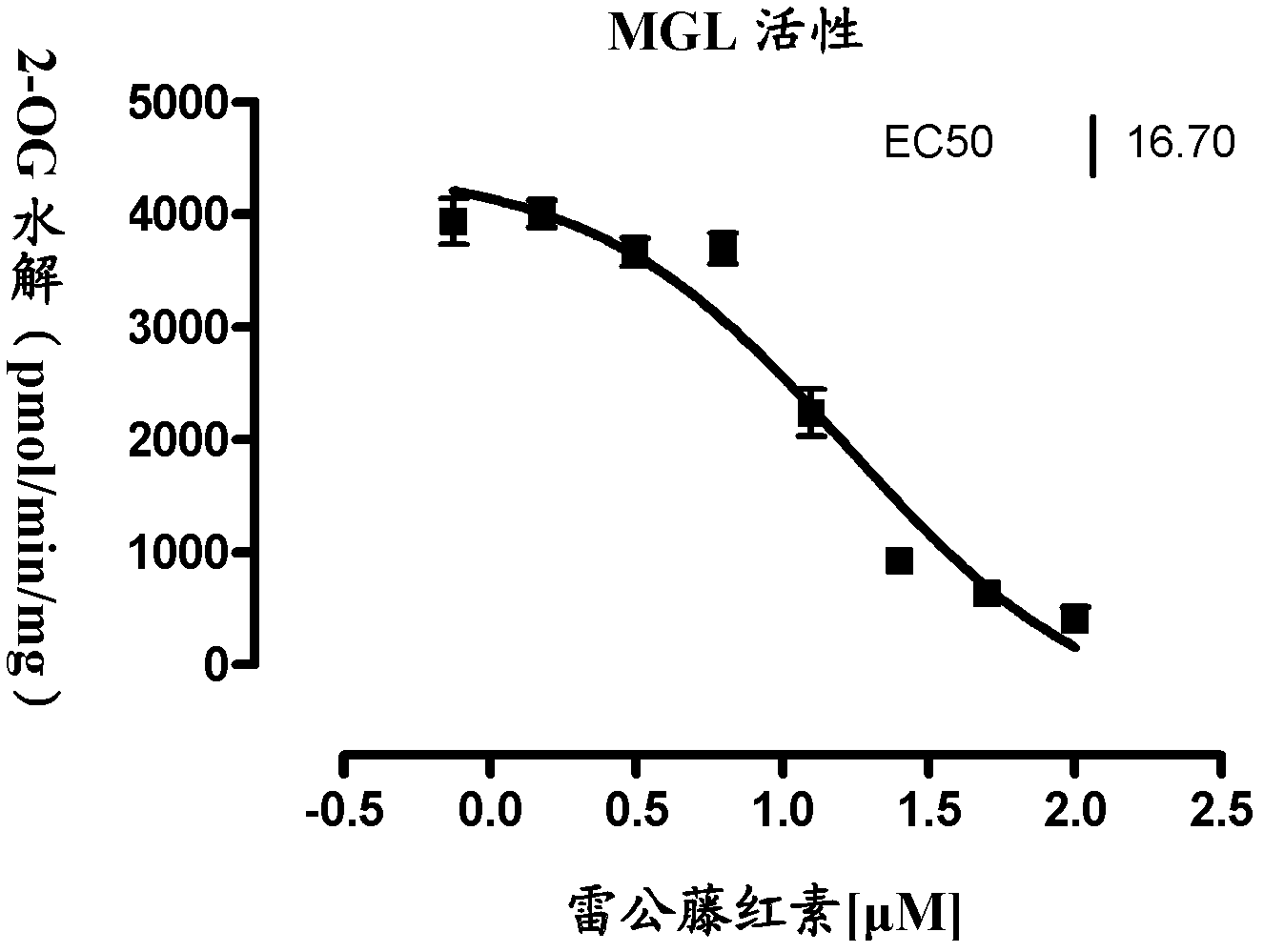
[0035] FAAH, MGL enzyme inhibitory activity detection
[0036] Add 30 μL (1 mg / mL) protein into the injection bottle, then add 2 μL DMSO (blank control group) or different concentrations of tripterine and react at 37 degrees Celsius for 10 minutes. Then add 170 μL of buffer solution containing substrate AEA or 2-OG (in the experiment, 2-OG was used to replace 2-AG, which is difficult to detect quantitatively, and this substitution is well known and allowed in the art), and the concentration of AEA is 5 μM. After reacting at 37° C. for 30 minutes, 200 μL of methanol solution containing heptadecanoic acid (internal standard) was added to terminate the reaction. The production of arachidonic acid and oleic acid in the hydrolyzate was detected by liquid chromatography-mass spectrometry (LC-MS), and graphed with Graphpad Prism 5. In this way, the EC50 values obtained by measuring tripterine to FAAH and MGL enzymes are 4.69 and 16.70 μM respectively (see figure 1 and figure 2 ...
Embodiment 2
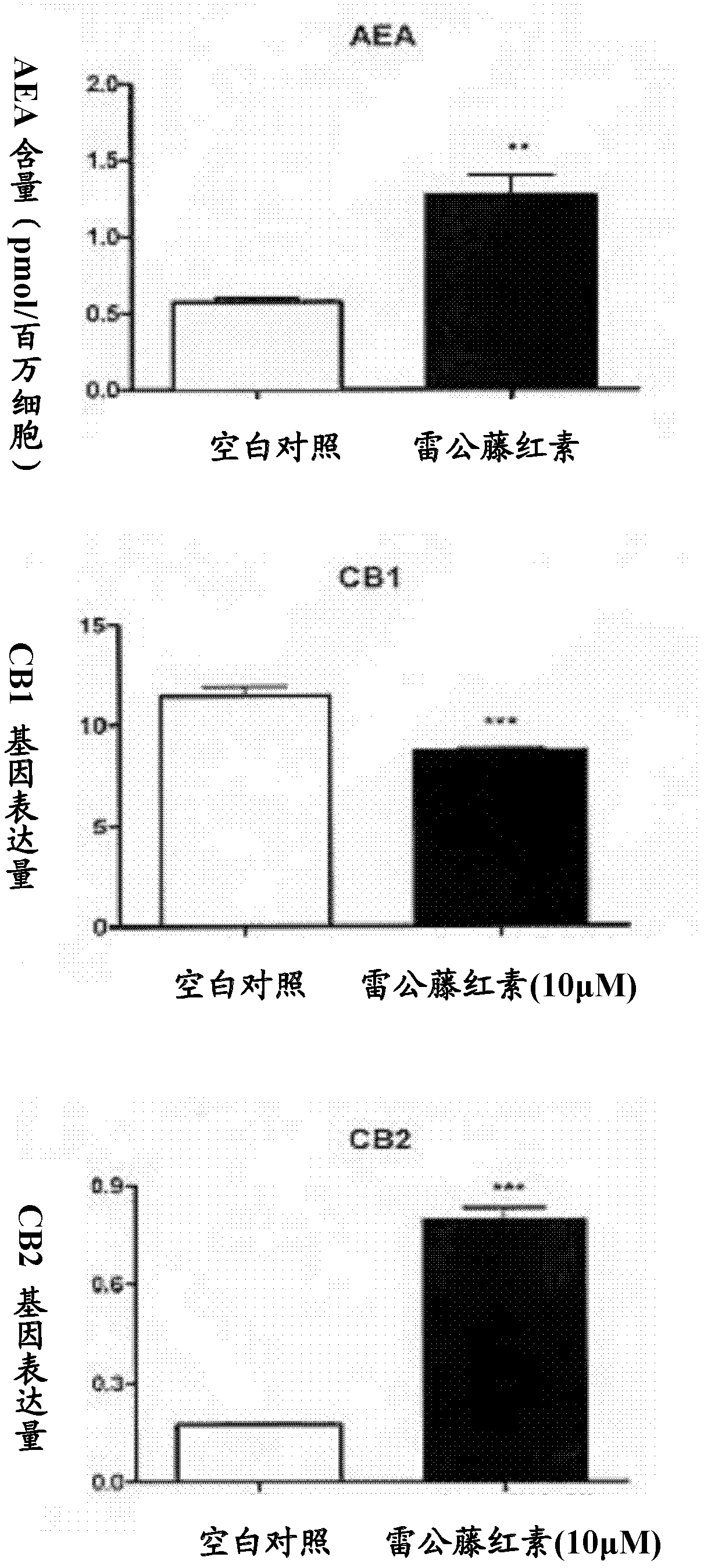
[0038] LC-MS detection of endocannabinoid content in cells
[0039] Four hours after cell administration, cells were harvested with methanol / water (1:1, v:v), sonicated and extracted with chloroform. The lower organic layer was collected by centrifugation at 3000 rpm, dried with nitrogen gas, redissolved with 100 μL of 3:1 methanol / chloroform, and then detected the content of endocannabinoids by LC-MS. This experiment showed that after induction by tripterine, the expression of AEA in cells was significantly increased (see image 3 ).
Embodiment 3
[0041] Cytotoxicity test
[0042]Glioma cells were inoculated in a 96-well plate at a concentration of 5000 cells / well, and culture medium containing different concentrations of tripterygium wilfordii was replaced the next day after the cells adhered to the wall for 24 hours. Then add 10 μL of cell counting reagent (CCK-8), continue to incubate for 2 hours, and then measure the absorbance with a 450 nm microplate reader. The number of dead cells was calculated with the blank group as 100%, and the EC50 value of tripterine on various glioma cells was preliminarily predicted based on the curve. Experiments have shown that the EC50 values of tripterine to C6, BT325, SHG44 and other glioma cells are between 2.0-3.5 μM, showing good cytotoxic activity (see Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com