Copper-based catalyst used in preparation of glycol by catalytic hydrogenation of oxalate ester and preparation method thereof
A technology for copper-based catalysts and hydrogen preparation, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve instability, loss of activity, poor catalyst stability, etc. problem, to achieve the effect of high stability, high selectivity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
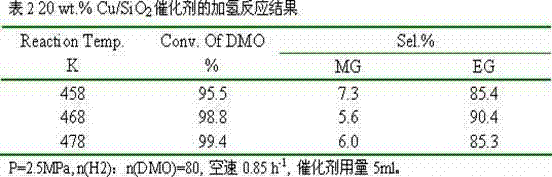
[0016] Dissolve 1.13g of copper nitrate and 0.28g of polyvinylpyrrolidone 4000 in 500ml of absolute ethanol, transfer them to a Teflon-lined reactor, and react at 300°C for 4 hours. After cooling, transfer it to a beaker to obtain an ethanol suspension of copper oxide nanoparticles. Then add 800 ml of absolute ethanol, 1.0 L of deionized water, 50 ml of ammonia water, and 5 g of cetyltrimethylammonium bromide to the above suspension, stir evenly, and then add 10 ml of ethyl orthosilicate. Reacted for 12 hours, filtered, dried, and calcined at 500°C for 3 hours to obtain a copper-based catalyst with a core-shell structure, labeled as 10 wt.% Cu / SiO 2 .
[0017] The catalyst is granulated into 60-80 meshes, with 10% H 2 / N 2 Activity evaluation was carried out after in situ reduction, and the hydrogenation of oxalate to ethylene glycol was carried out. The results of selective hydrogenation are shown in Table 1.
[0018]
Embodiment 2
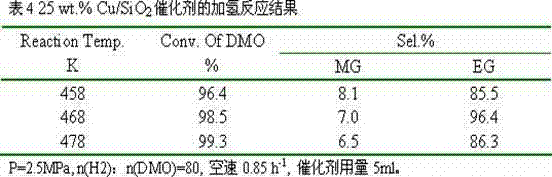
[0020] 3.82g of copper nitrate and 1.91g of polyvinylpyrrolidone 10000 were dissolved in 1000ml of absolute ethanol respectively, transferred to a Teflon-lined reactor, and reacted in a homogeneous reactor at 400°C for 5 hours. After cooling, transfer it to a beaker to obtain an ethanol suspension of copper oxide nanoparticles. Then add 1000ml of absolute ethanol, 1500ml of deionized water, 100ml of ammonia water, and 8g of cetyltrimethylammonium bromide to the above suspension, stir well and then add 15ml of ethyl orthosilicate. Reacted for 20 hours, filtered, dried, and calcined at 400°C for 4 hours to obtain a copper-based catalyst with a core-shell structure, marked as 20 wt.% Cu / SiO 2 .
[0021] The catalyst is granulated into 60-80 meshes, with 10% H 2 / N 2 Activity evaluation was carried out after in situ reduction, and the hydrogenation of oxalate to ethylene glycol was carried out. The results of selective hydrogenation are shown in Table 2.
[0022]
Embodiment 3
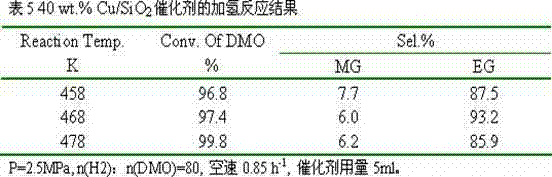
[0024] 8.73g of copper nitrate and 6.55g of polyvinylpyrrolidone 80000 were dissolved in 800ml of absolute ethanol respectively, transferred to a polytetrafluoroethylene-lined reactor, and reacted at 350°C for 4 hours. After cooling, transfer it to a beaker to obtain an ethanol suspension of copper oxide nanoparticles. Then add 2L of absolute ethanol, 1.6L of deionized water, 150ml of ammonia water, and 6g of cetyltrimethylammonium bromide to the above suspension, stir well and then add 10ml of ethyl orthosilicate. Reacted for 30 hours, filtered, dried, and calcined at 500°C for 6 hours to obtain a copper-based catalyst with a core-shell structure, marked as 30 wt.% Cu / SiO 2 .
[0025] The catalyst is granulated into 60-80 meshes, with 10% H 2 / N 2 Activity evaluation was carried out after in situ reduction, and the hydrogenation of oxalate to ethylene glycol was carried out. The results of selective hydrogenation are shown in Table 3.
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com