Reactive halogen-free flame retardant and preparation method thereof
A reactive, flame retardant technology, applied in the field of flame retardants, can solve the problems of material physical, mechanical and processing properties being greatly affected, and achieve the effect of easy operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
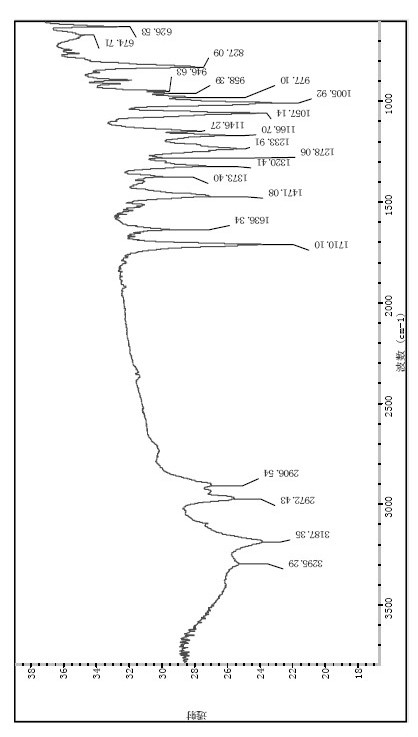
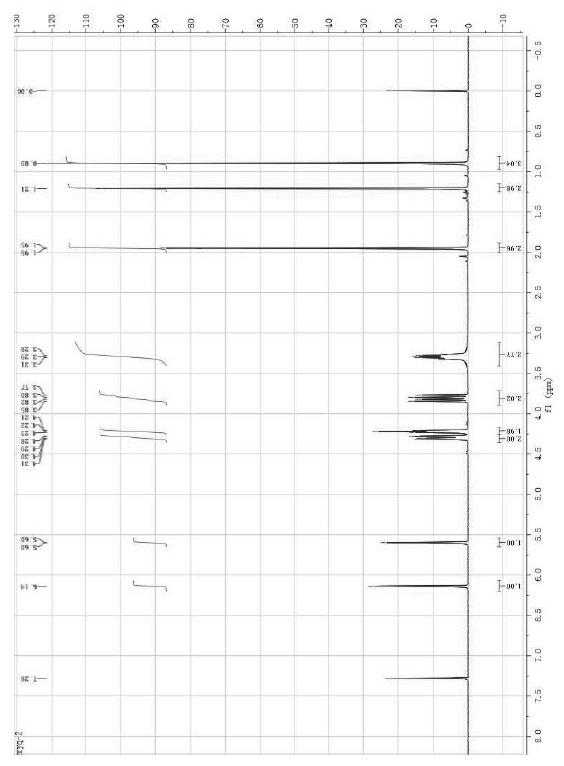
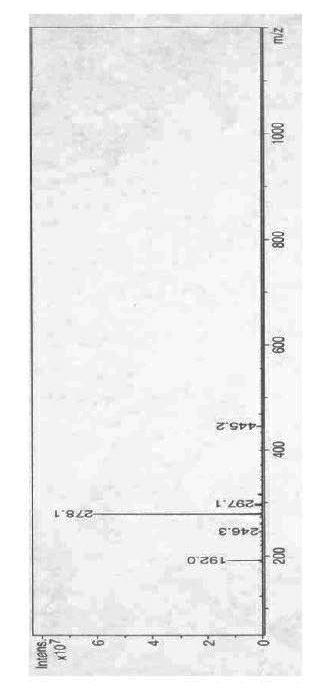
[0031] In a 250 mL three-necked flask equipped with stirring, reflux condenser and thermometer, 12.9 g (0.10 mol) of aminoethyl methacrylate, 12.0 g (0.12 mol) of triethylamine, 0.1 g of hydroquinone and 80 mL of tetrahydrofuran, stirred at room temperature for 0.5 h, then added 18.5 g (0.10 mol) of cyclic neopentyl glycol phosphoryl chloride and 0.7 g of 4-(N,N-dimethylamino)pyridine, adjusted the temperature to about 50 °C, The stirring reaction was continued for 4.5 h. The insoluble matter was removed by filtration under reduced pressure, the filtrate was distilled under reduced pressure and tetrahydrofuran was recovered, the sticky matter in the bottle was adjusted to neutrality with 5% dilute hydrochloric acid, washed with 2×50 mL water, and recrystallized with ethyl acetate / petroleum ether to obtain White to light red needle-like crystals, after drying, 22.3 g of the product was obtained, with a yield of 80.5%. The melting point of the product is 77.5-78.5°C, and its in...
Embodiment 2
[0033] In a 250 mL three-neck flask equipped with stirring, reflux condenser and thermometer, add 16.6 g (0.10 mol) of aminoethyl methacrylate hydrochloride, 19.0 g (0.24 mol) of pyridine, and 0.2 g of hydroquinone , 75 mL of ethyl acetate, stirred at room temperature for 0.5 h, then added 15.9 g (0.08 mol) of cyclic neopentyl glycol phosphorus oxychloride and 0.8 g of anhydrous magnesium chloride, adjusted the temperature to about 60 °C, and continued to stir for 10 h. Post-processing and structural characterization are the same as in Example 1. 21.5 g of the product was obtained, and the yield was 77.6%.
Embodiment 3
[0035] In a 250 mL three-necked flask equipped with stirring, reflux condenser and thermometer, add 11.5 g (0.10 mol) of aminoethyl acrylate, 11.0 g (0.11 mol) of triethylamine, 0.2 g of hydroquinone, and 50 mL of chloroform , stirred at room temperature for 0.5 h, then added 18.4 g (0.10 mol) of cyclic neopentyl glycol phosphorus oxychloride and 1.0 g of anhydrous magnesium chloride, adjusted the temperature to about 65 °C, and continued to stir for 15 h. After filtration, the filtrate was distilled and the solvent was recovered. The sticky matter in the bottle was fully washed with 2×50 mL of water, recrystallized with ethanol / water, and dried to obtain 20.6 g of the product, with a yield of 78.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com