Near infrared fluorescent dye, preparation and application thereof
A fluorescent dye, near-infrared technology, used in azo dyes, organic dyes, luminescent materials, etc., can solve the problems of lower detection limit and low detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, the preparation of important precursor
[0028] Compound 1:
[0029] P-bromoacetophenone (9.9g, 50mmol) and potassium hydroxide (8.4mg, 1.5mmol) were added to a round bottom flask, all dissolved in ethanol / water solution (85:15v / v 100mL), and then thiophenecarbaldehyde ( 5.6g, 50mmol), stirred at room temperature for 24h, during this reaction process, the product precipitated from the mixed solution, after the reaction was over, the mixed solution was filtered and recrystallized with ethanol to obtain a yellow solid (13.5g, 92.5%), GC- MS (EI-m / z): theoretical, 291.96; found, 292.
[0030]
[0031] Compound 2:
[0032] Compound 1 (4.9g, 16.8mmol) was weighed and dissolved in 100mL of methanol, nitromethane (5.13g, 84mmol) and diethylamine (6.15g, 84mmol) were added, and heated to reflux for 24h. After the reaction is complete, cool and stand still, acidify to weak acidity with 1mol / L hydrochloric acid, extract and separate the liquids with dichlorome...
Embodiment 2
[0037] Embodiment 2, preparation of aza-BODIPY near-infrared material
[0038] Compound 4a:
[0039]Weigh substance 3 (100mg, 0.15mmol), fluorene monoboronate (164mg, 0.69mmol) and triphenylphosphorous palladium catalyst (7.8mg) into a 25mL two-necked bottle, vacuum blow nitrogen, and inject into the two-necked bottle Toluene (1.5 mL), ethanol (0.75 mL) and 2M potassium carbonate aqueous solution (0.75 mL) were preliminarily sparged with nitrogen for 30 minutes, and heated under reflux overnight. After the reaction was completed, it was cooled to room temperature, extracted with dichloromethane, and the organic layer was spin-dried and passed through a column (PE:DCM=6:1) to obtain a dark purple solid (94.8mg, 48.6%). 1 H NMR (400MHz, CDCl 3 )δ: 8.20 (d, J = 8.2Hz, 4H), 7.97 (d, J = 3.2Hz, 2H), 7.85-7.71 (m, 8H), 7.69-7.62 (m, 4H), 7.59 (d, J =4.8Hz, 2H), 7.40-7.30(m, 6H), 7.25-7.19(m, 2H), 7.03(s, 2H), 2.12-1.93(m, 8H), 1.33-0.99(m, 40H), 0.81 (t, J = 6.9 Hz, 12H) 0.74-0....
Embodiment 3
[0044] Embodiment 3, substituting group is the aza BODIPY of fluorene to Hg in solution 2+ Titration experiment
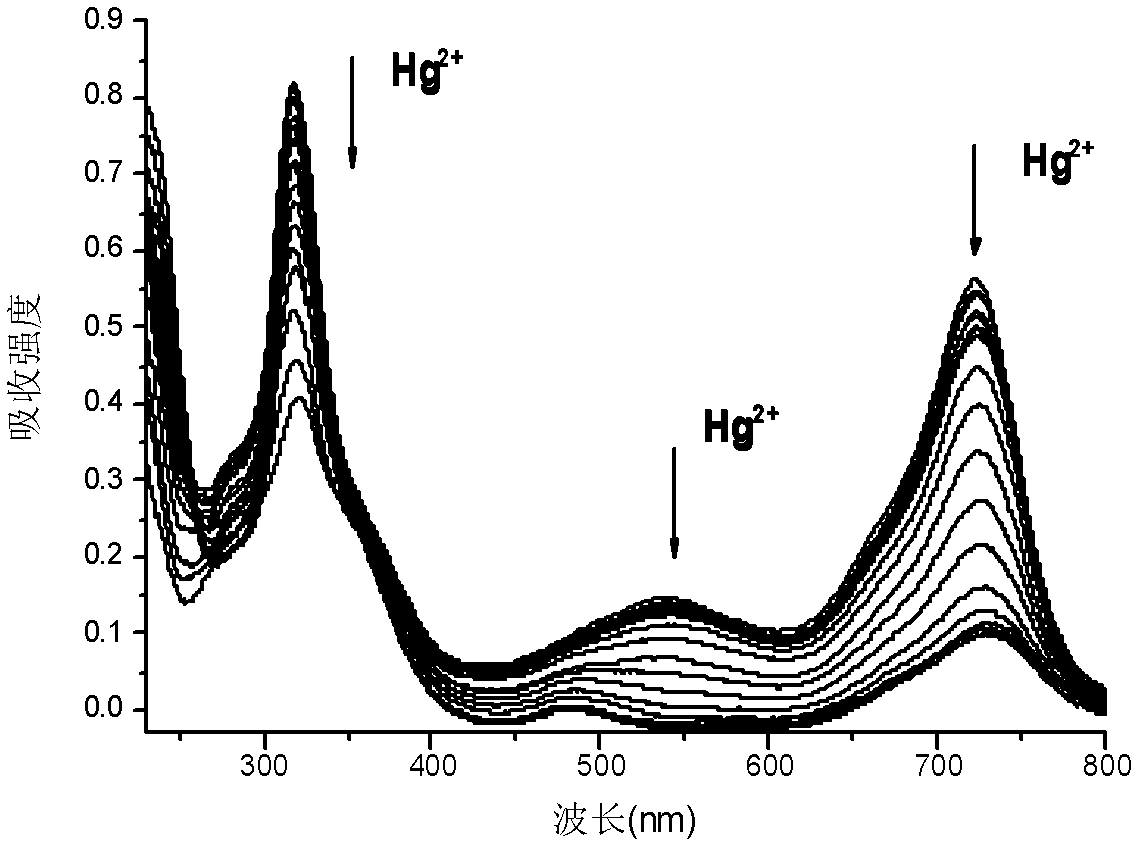
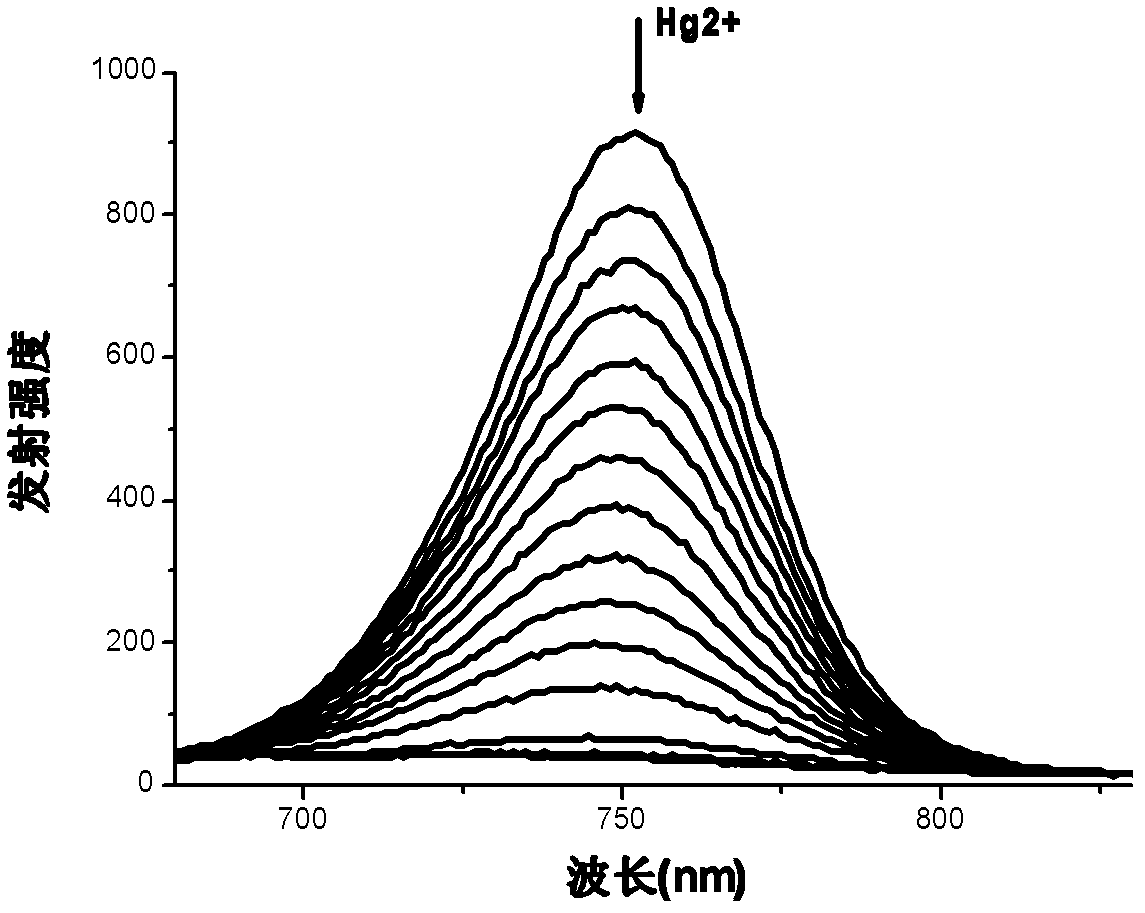
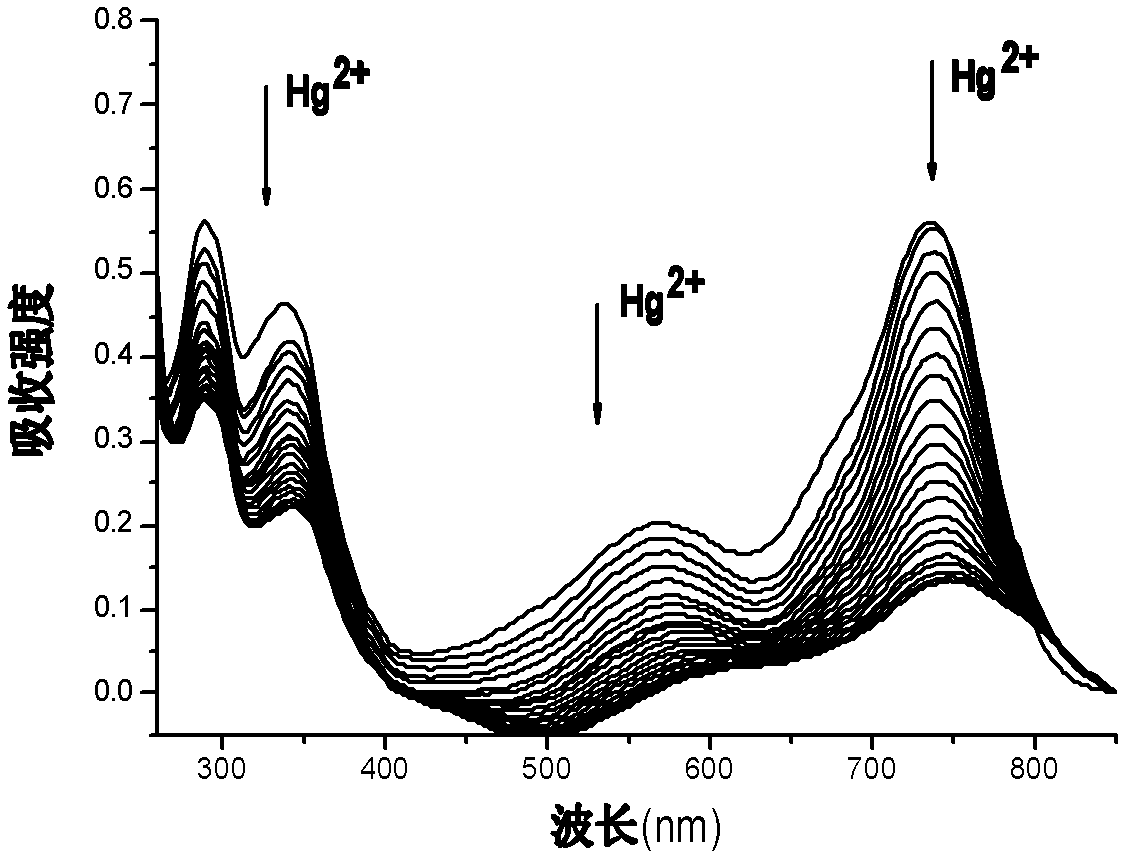
[0045] Prepare a 10 μM solution of azaBODIPY (4a) whose substituent is fluorene (HPLC dichloromethane as a solvent), pipette 2.5 mL of the compound solution into a fluorescent cuvette, and gradually add 1.25×10 -3 mol / LHg 2+ solution (acetonitrile as solvent), measure its ultraviolet-visible absorption spectrum and fluorescence spectrum respectively until the spectrum reaches equilibrium (that is, the spectrum no longer changes significantly). Test data show that with Hg 2+ With the addition of , the color of the compound solution changed significantly, from light purple to light blue, and the fluorescence was gradually quenched.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com