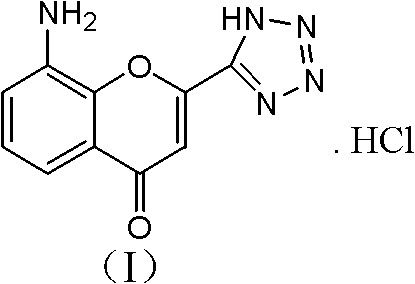
Preparation method of 8-amino-2-(1H-tetrazol-5-yl)-chromen hydrochloride
A technology of benzopyrone hydrochloride and hydroxyacetophenone, applied in the field of medicine and chemical industry, can solve the problems of difficult realization of industrialized production, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of N-(3-acetyl-2-hydroxyl-phenyl)-acetamide (III)
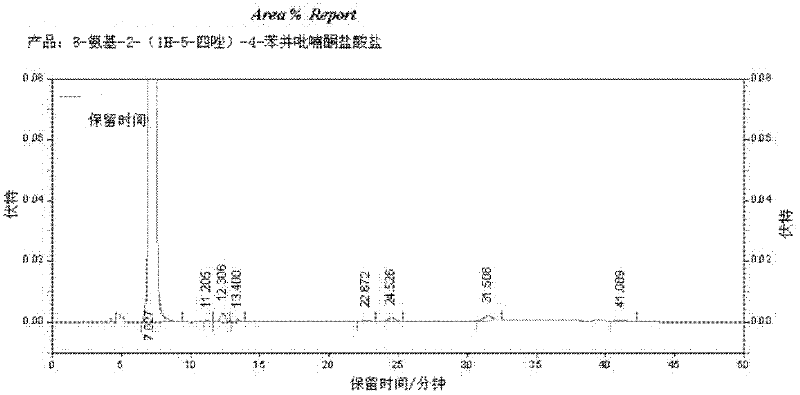
[0027] Install a thermometer and a mechanical stirrer on a 500ml four-neck flask, and connect a tail gas absorption device. Then, 30.2 g (0.2 mol) of 3-amino-2-hydroxyacetophenone (II), 240 ml of ethyl acetate, 30 g of 20% aqueous sodium hydroxide solution and 16 g (0.2 mol) of pyridine were added to the flask. Stirring was started, and the reaction temperature was controlled at 0-5°C, and 16 g (0.2 mol) of acetyl chloride was added dropwise, and the addition was completed within 0.5 h. The reaction was incubated for 1 h, and samples were taken for liquid chromatography analysis to determine the end point of the reaction. Warm up to 20-30°C, add 10ml of water and 12.5ml of concentrated hydrochloric acid (analytical pure), and continue stirring for 0.5h. The sodium hydroxide aqueous solution is used to neutralize the hydrochloride, and water and concentrated hydrochloric acid are added to wash the reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com