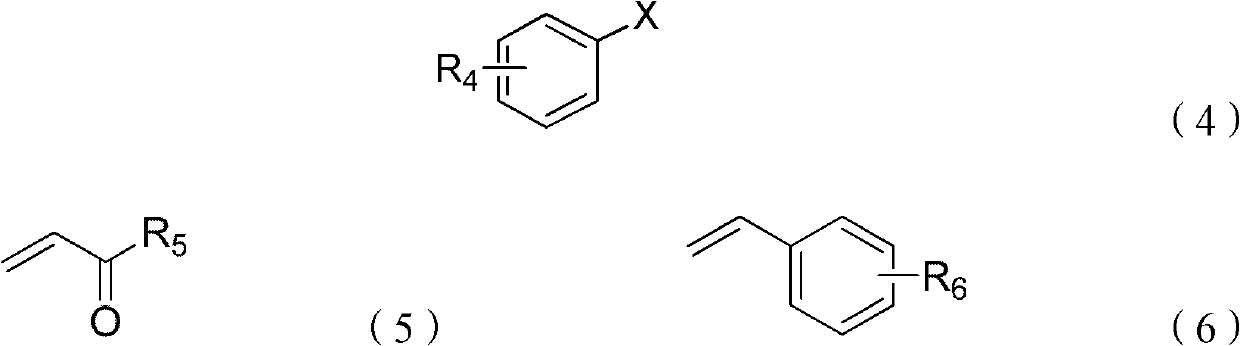
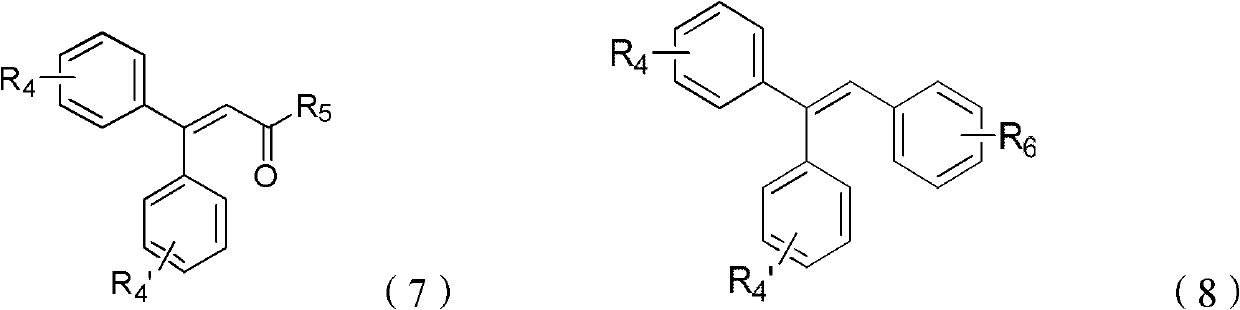
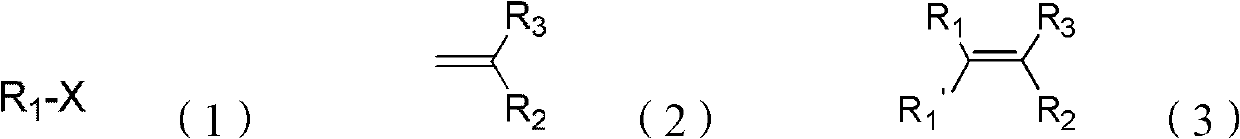Synthesis method of beta, beta-diaryl alkene
A diarylene and synthesis method technology, applied in the field of β, can solve the problems of alkali failure reaction, difficult to occur, etc., and achieve the effect of mild reaction conditions, less catalyst consumption, and good reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 prepares β by iodobenzene and ethyl acrylate, and β-diphenyl acrylate ethyl ester
[0041] Add 1.2mg (0.005mmol) of palladium acetate, 173mg (1.05mmol) of silver acetate, 1.5mL of glacial acetic acid, 0.408g (2mmol) of iodobenzene, and 50mg (0.5mmol) of ethyl acrylate into a 25mL reaction vessel with a stirring magnet. In the tube, stir and heat, and react at 110°C for 4 hours; after the reaction, cool, add 10 mL each of ethyl acetate and dichloromethane, filter, spin the filtrate to dry and concentrate, and separate the product β, β-diphenyl Ethyl acrylate 118 mg, yield 94%. 1 H NMR (400MHz, CDCl 3 , ppm): δ7.43-7.41 (m, 3H), 7.38-7.37 (m, 1H), 7.35-7.34 (m, 4H), 7.26-7.25 (m, 2H), 6.42 (s, 1H), 4.10 (q, J=7.2Hz, 2H), 1.15(t, J=7.2Hz, 3H). 13 C NMR (100MHz, CDCl 3 , ppm): δ166.1, 156.5, 140.8, 139.0, 129.4, 129.1, 128.4, 128.3, 128.1, 127.9, 117.5, 60.0, 13.0.IR: 2980, 1719, 1616, 1491, 1445, 1368, 1262, 1154, 1033, 865, 770, 695, 615cm -1 .MS (EI, m...
Embodiment 2
[0042] Embodiment 2 prepares β by p-methyl iodobenzene and ethyl acrylate, and β-two-(p-methylphenyl) ethyl acrylate
[0043] 1.2mg (0.005mmol) of palladium acetate, 173mg (1.05mmol) of silver acetate, 1.5mL of glacial acetic acid, 0.436g (2mmol) of p-methyliodobenzene, and 50mg (0.5mmol) of ethyl acrylate were successively added to the In a 25mL reaction tube, stir and heat, and react at 110°C for 4 hours; after the reaction, cool, add 10mL each of ethyl acetate and dichloromethane, filter, spin the filtrate to dryness and concentrate, and separate the product β, β- Di-(p-methylphenyl) ethyl acrylate 136 mg, yield 97%. 1 H NMR (400MHz, aceton-d 6 , ppm): δ7.17-7.11 (m, 6H), 7.01-6.99 (d, J = 8.0Hz, 2H), 6.24 (s, 1H), 3.94 (q, J = 7.1Hz, 2H), 2.32 ( s, 3H), 2.28(s, 3H), 1.03(t, J=7.2Hz, 3H). 13 C NMR (100MHz, CDCl 3 , ppm): δ166.2, 1569, 139.6, 138.3, 137.9, 136.1, 129.2, 129.0, 128.5, 128.3, 116.2, 59.9, 21.4, 21.2, 14.1.IR: 2980, 1719, 1605, 1510, 1447, 1368, 1263, 1150...
Embodiment 3
[0044] Embodiment 3 prepares β by m-methyl iodobenzene and ethyl acrylate, and β-two-(m-methylphenyl) ethyl acrylate
[0045] Add 1.2mg (0.005mmol) of palladium acetate, 173mg (1.05mmol) of silver acetate, 1.5mL of glacial acetic acid, 0.436g (2mmol) of m-methyliodobenzene, and 50mg (0.5mmol) of ethyl acrylate into the In a 25mL reaction tube, stir and heat, and react at 110°C for 4 hours; after the reaction, cool, add 10mL each of ethyl acetate and dichloromethane, filter, spin the filtrate to dryness and concentrate, and separate the product β, β- Di-(m-methylphenyl) ethyl acrylate 129 mg, yield 92%. 1 H NMR (400MHz, CDCl 3 , ppm): δ7.34-7.30 (t, 1H), 7.28-7.19 (m, 4H), 7.14-7.12 (d, 1H), 7.08-7.06 (m, 2H), 6.38 (d, J=0.8, 1H), 4.11(q, J=7.2, 2H), 2.40(s, 3H), 2.37(s, 3H), 1.17(t, J=7.2Hz, 3H). 13 C NMR (100MHz, CDCl 3 , ppm): δ166.2, 156.8, 140.9, 139.0, 137.9, 137.3, 130.1, 129.6, 128.8, 128.2, 127.6, 126.3, 125.6, 117.2, 59.9, 21.4, 21.3, 14.0. IR: 2979, 17321, 160 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com