Copolymer containing fluorine and thiophene pyrroledione unit as well as preparation method and application thereof
A technology of thienopyrrole diketone and copolymer, which is applied in the direction of chemical instruments and methods, electrical components, and the structure of active regions, and can solve the problems of low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
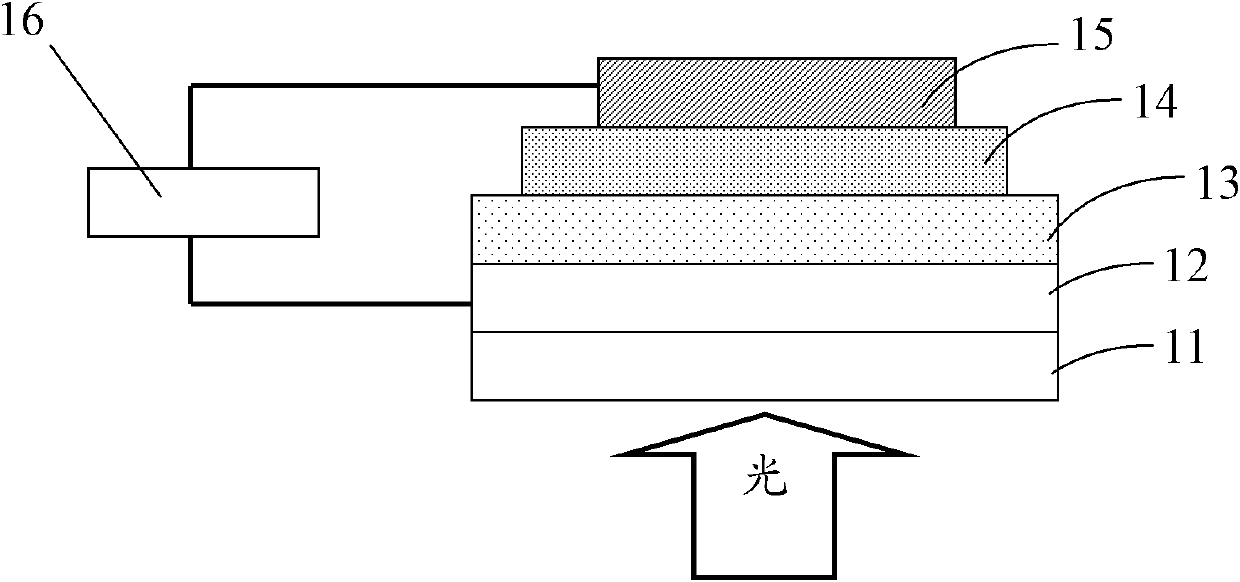
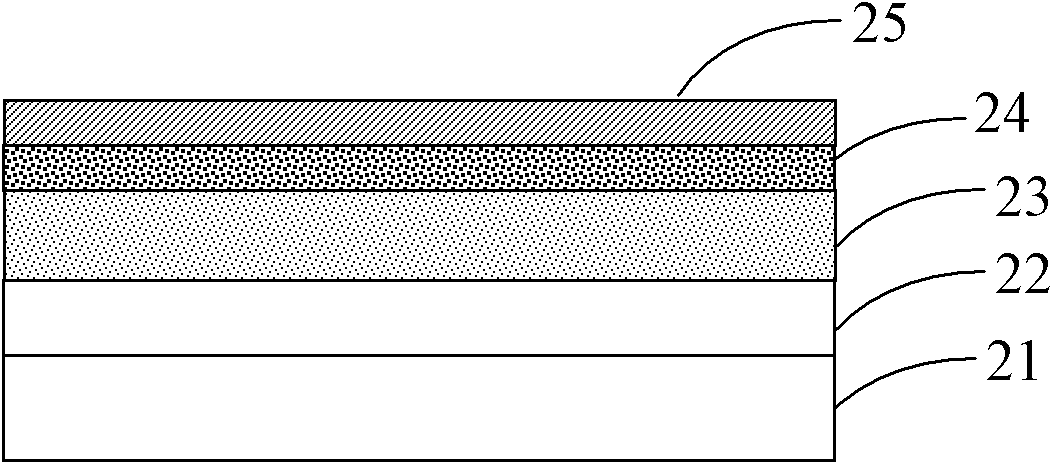
Image
Examples
preparation example Construction
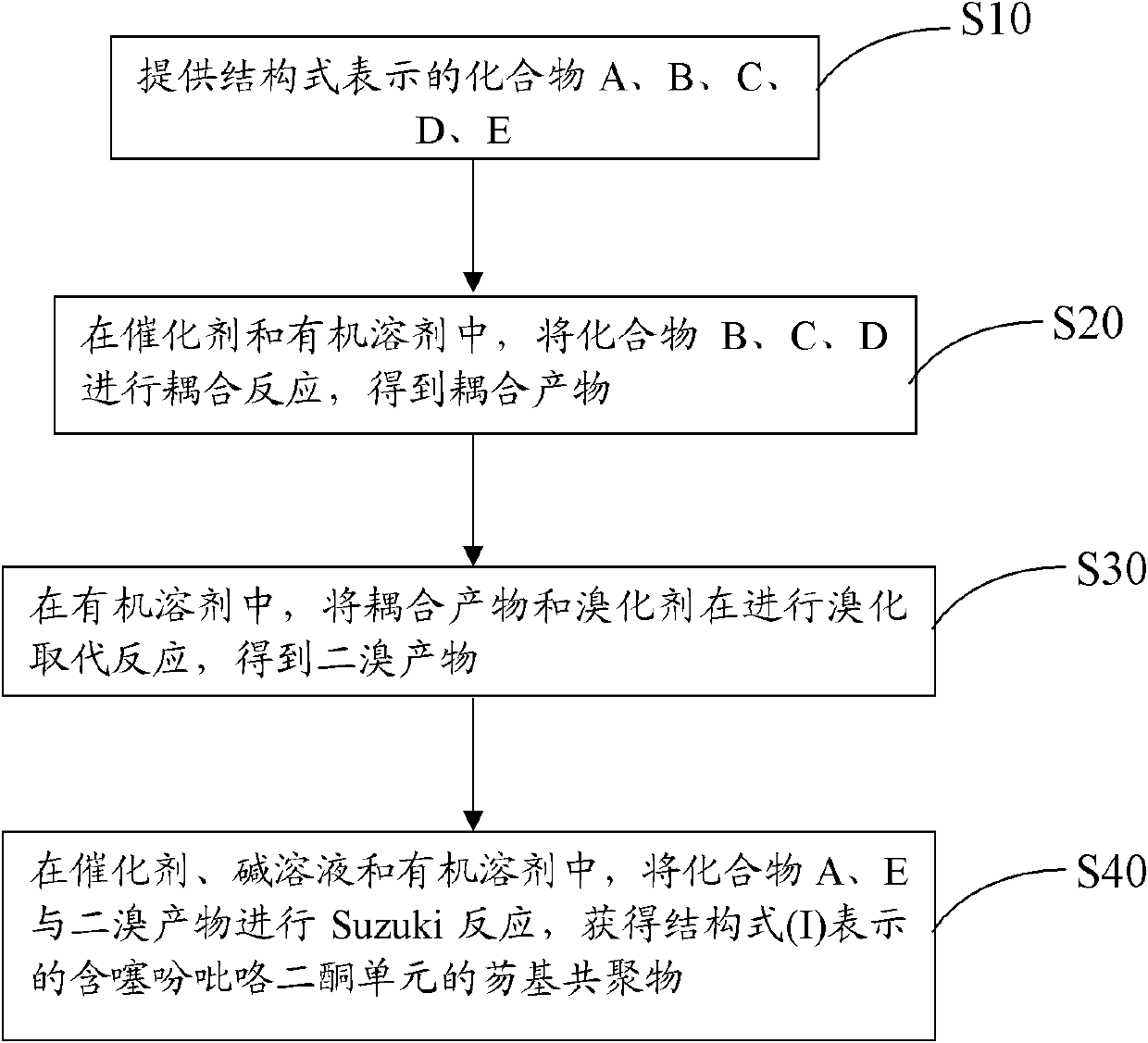
[0033] see figure 1 , the preparation method of the above-mentioned copolymer containing fluorene and thiophene pyrrole diketone unit comprises the steps:
[0034] S10: provide compounds A, B, C, D, E represented by the following structural formula,
[0035] Where: R 1 , R 2 , R 3 are identically or differently denoted as C 1 -C 20 the alkyl group; R 4 , R 5 , R 6 , R 7 denoted H or C for the same or different 1 -C 20 the alkyl group;
[0036] S20: In a catalyst and an organic solvent, compounds B, C, and D are subjected to the coupling reaction shown in the following formula to obtain a coupled product,
[0037]
[0038] S30: In an organic solvent, the coupling product and the brominating agent are subjected to a bromination substitution reaction to obtain a dibrominated product;
[0039] S40: In catalyst, alkali solution and organic solvent, carry out Suzuki reaction with compound A, compound E and dibromo product, obtain the copolymer containing fluorene...
Embodiment 1
[0068] In the copolymer (I1) containing fluorene and thiophene pyrrole diketone units of this embodiment, R 1 , R 2 , R 3 Both are methyl, R 4 , R 5 , R 6 , R 7 All are H, x=1 / 4, y=3 / 4, n=20, and its structural formula is as follows:
[0069]
[0070] The copolymer represented by the above structural formula (I 1 ), R 4 , R 5 , R 6 , R 7 Both are H, indicating that compounds C and D have the same structure, so only one raw material needs to be provided, which not only simplifies the source of raw materials, but also improves the reaction yield.
[0071] The copolymer of the present embodiment (I 1 ) preparation comprises the following specific steps:
[0072] 1. The preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxabororyl)-9,9-dimethylfluorene, i.e. compound A A specific example, its structural formula is as follows:
[0073]
[0074] The specific preparation process is as follows: Add 20.00mL (1.00M) n-butyllithium solution to a solution containing 3....
Embodiment 2
[0093] The copolymer (I 2 ), R 1 , R 2 , R 3 Both are C 8 h 17 ,R 4 , R 5 , R 6 , R 7 All are H, x=1 / 2, y=1 / 2, n=44, and its structural formula is as follows:
[0094]
[0095] Similar to Example 1, the copolymer represented by the above structural formula (I 2 ), R 4 , R 5 , R 6 , R 7 Both are H, indicating that compounds C and D have the same structure, so only one raw material needs to be provided, which not only simplifies the source of raw materials, but also improves the reaction yield. Moreover, R 1 , R 2 , R 3 and R 8 , R 9 Both are C 8 h 17 , which can improve the copolymer (I 2 ) molecular weight, which is conducive to processing and forming a film, for example, it is convenient for the copolymer to form a film by spin coating technology.
[0096] The copolymer of the present embodiment (I 2 ) preparation comprises the following specific steps:
[0097] One, the preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com