Indole derivative with pyrrolidone structure and preparation method thereof
A technology of indole derivatives and pyrrolidone, which is applied in the field of indole derivatives, can solve the problems of increasing production costs and infeasibility, and achieve the effects of avoiding subsequent processing, low price and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
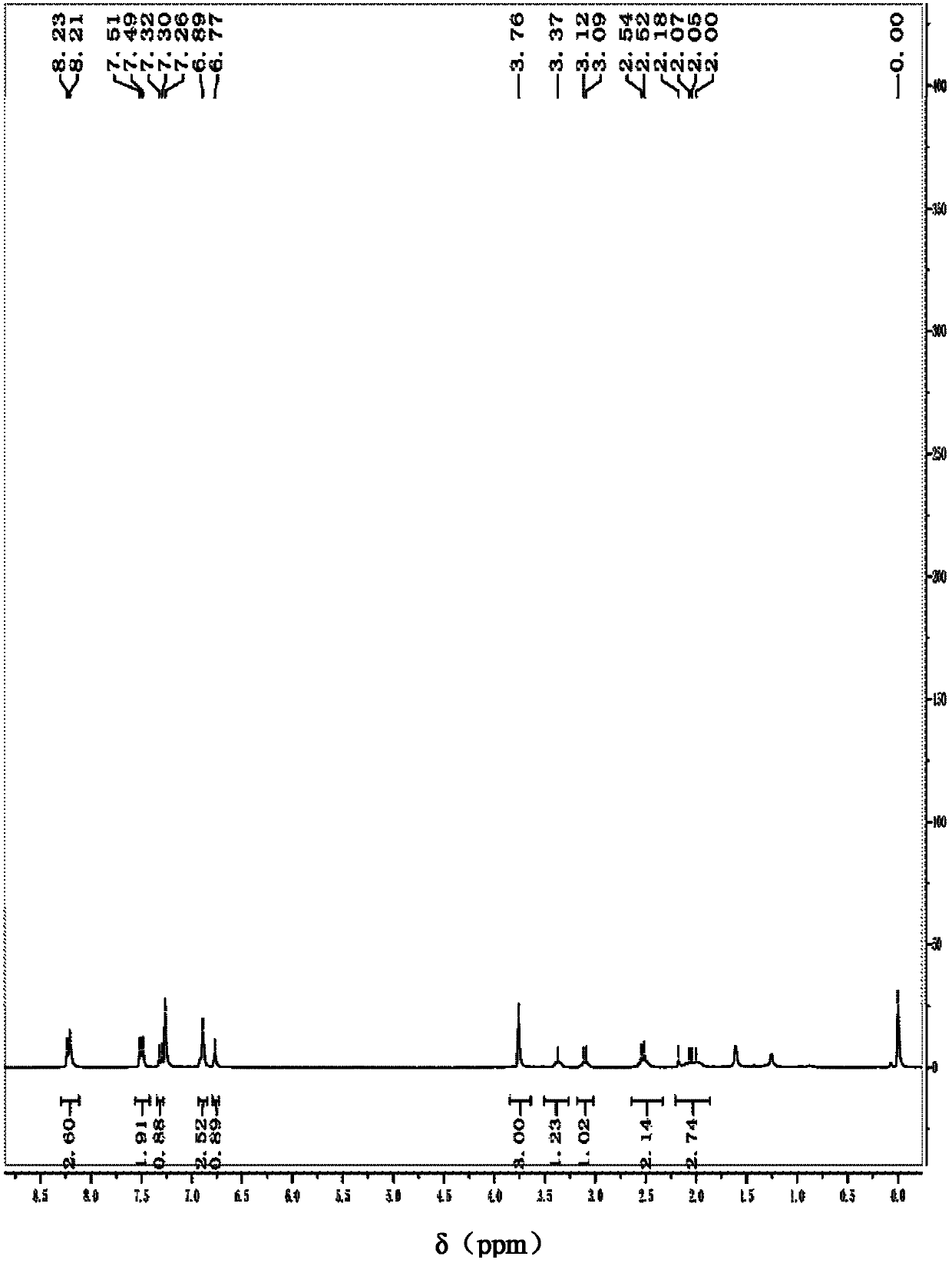
Image
Examples
preparation example Construction
[0041] The present invention also provides a method for preparing an indole derivative containing a pyrrolidone structure, comprising the following steps:
[0042] The indole compound with the structure of formula (V) and the pyrrolidone compound with the structure of formula (VI) react in an organic solvent under the catalysis of elemental iodine to obtain the indole with the structure of pyrrolidone with the structure of formula (IV) derivative;
[0043]
[0044] Wherein, R is hydrogen, alkyl, alkoxy, nitro, halogen, cyano or methylsulfonyl; R 1 is methyl, ethyl, n-propyl or isopropyl; R 2is hydrogen, alkyl, aryl, nitro, halogen, alkoxy or benzyloxy.
[0045] In the present invention, the indole compound with the structure of formula (V) and the pyrrolidone compound with the structure of formula (VI) are used as raw materials, and elemental iodine is used as the catalyst, and the compound with the structure of formula (IV) can be obtained by one-step reaction in an orga...
Embodiment 1
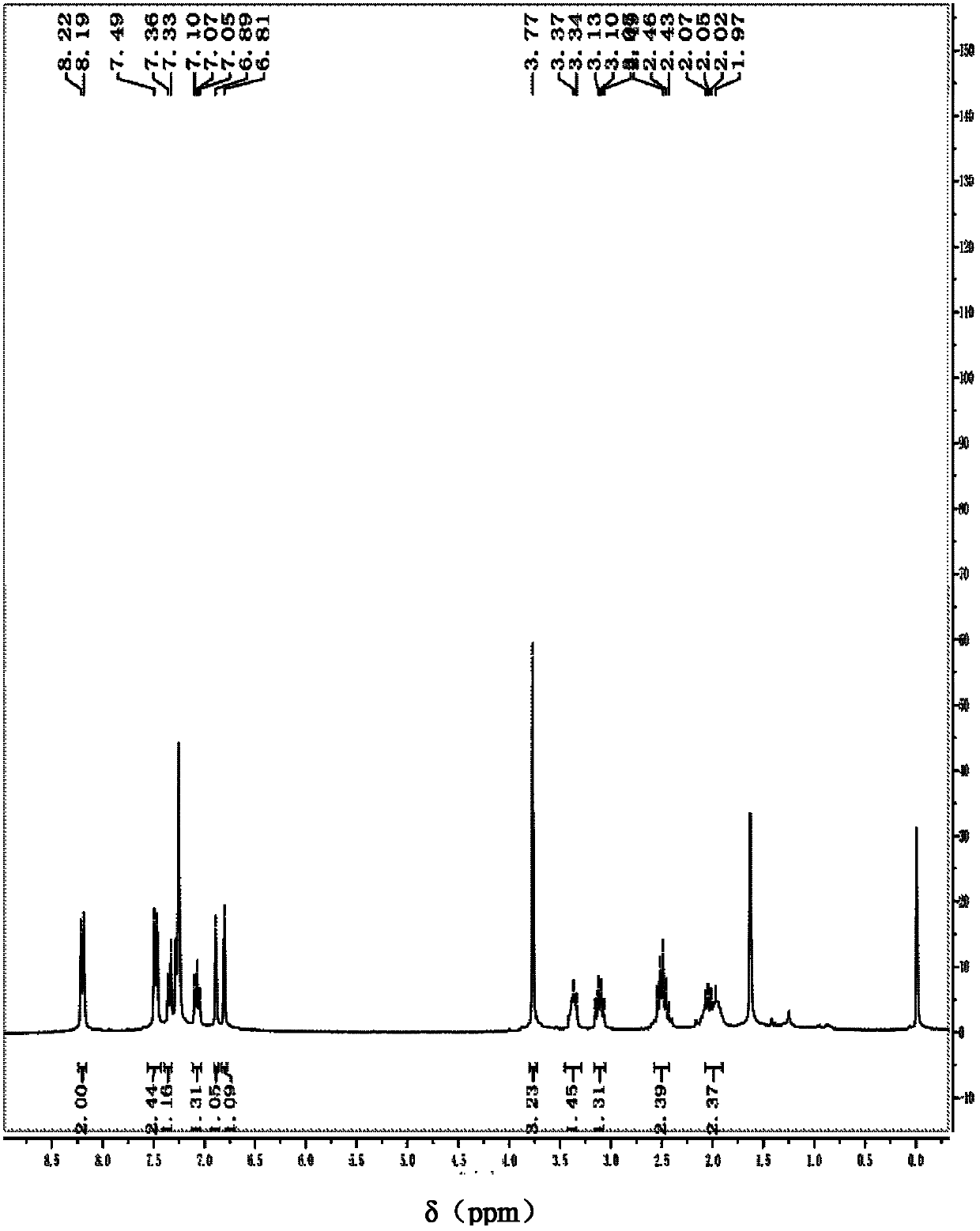
[0074] In a 25mL round bottom flask, add 0.1056g (0.4mmol) 1-ethoxyl (4-nitrophenyl) methyl) pyrrolidin-2-one, 0.0702g (0.6mmol) indole, 0.0050g (0.02mmol ) iodine and 2mL dichloromethane, stirred and refluxed at 40°C, detected by TLC (thin plate chromatography), and stopped after the disappearance of the raw material 1-ethoxy (4-nitrophenyl) methyl) pyrroline-2-one For the reaction, the reaction product was subjected to column chromatography to obtain 120.6 mg of 1-((1H-indol-3-yl)(4-nitrophenyl)methyl)pyrrolin-2-one with a yield of 90%.
[0075] The 1-((1H-indol-3-yl)(4-nitrophenyl)methyl)pyrroline-2-one is carried out to nuclear magnetic resonance analysis, and the spectrogram data obtained are as follows: 1 H NMR (300MHz, CDCl 3 ): δ=8.36(s, 1H), 8.21(d, J=8.6Hz, 2H), 7.49(d, J=8.6Hz, 2H), 7.42(d, J=8.1Hz, 1H), 7.31-7.22 (m, 3H), 7.11-7.06(t, J=7.5Hz, 1H), 6.97(s, 1H), 6.92(s, 1H), 3.42-3.35(m, 1H), 3.16-3.07(m, 1H ), 2.56-2.45 (m, 2H), 2.09-1.95 (m, 2H) ppm. From the ...
Embodiment 2
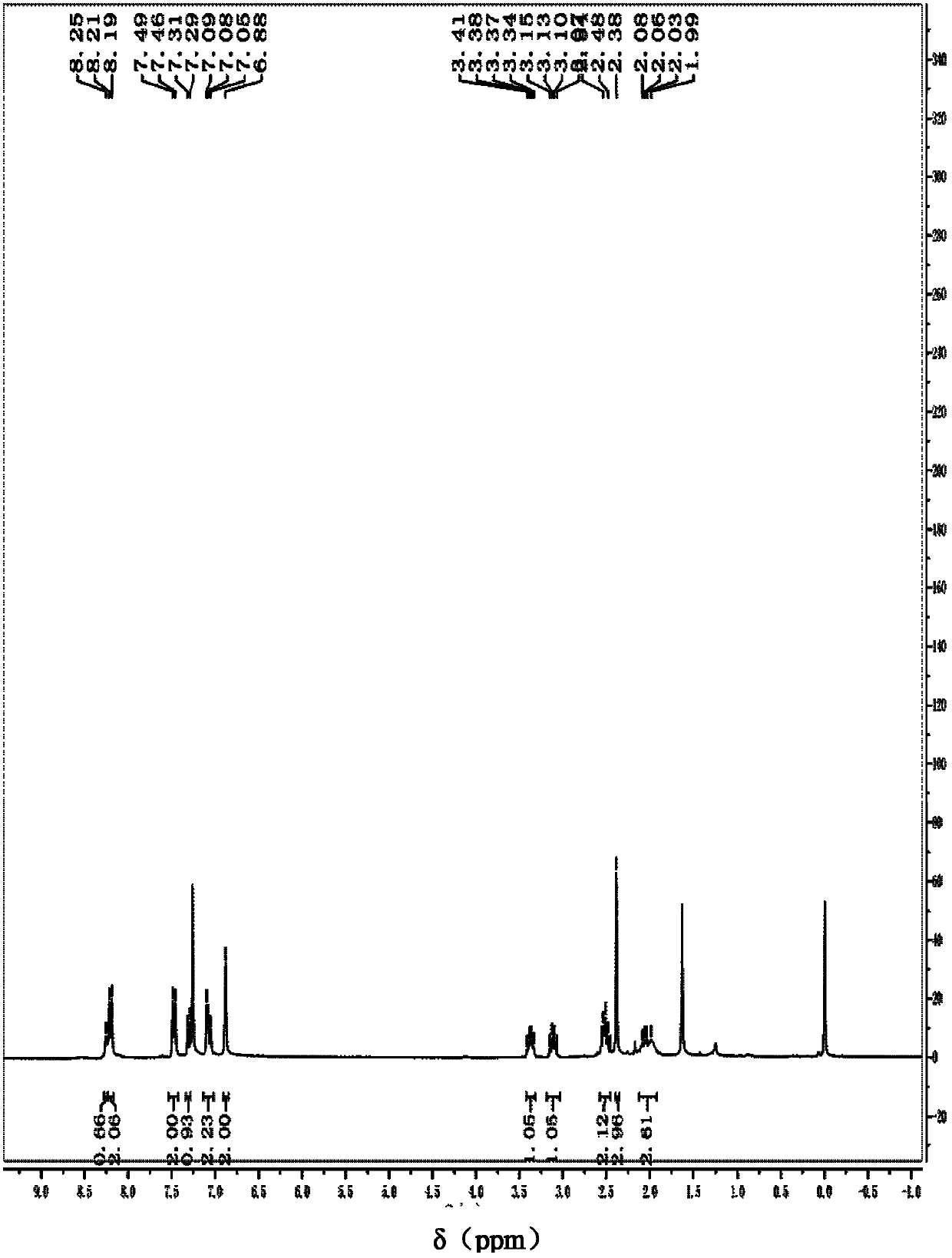
[0077] Add 0.1056g (0.4mmol) 1-ethoxy (4-nitrophenyl) methyl) pyrroline-2-one, 0.0787g (0.6mmol) 2-methyl indole, 0.005 g (0.02mmol) iodine and 2mL dichloromethane, stirred and refluxed at 40°C, detected by TLC (thin plate chromatography), the raw material 1-ethoxy (4-nitrophenyl) methyl) pyrroline-2- After the disappearance of the ketone, the reaction was terminated, and the reaction product was subjected to column chromatography to obtain 138 mg of 1-((2-methyl-1 hydrogen-indol-3-yl)(4-nitrophenyl)methyl)pyrroline-2 - Ketone, yield 99%.
[0078] The 1-((2-methyl-1 hydrogen-indol-3-yl) (4-nitrophenyl) methyl) pyrroline-2-one is carried out nuclear magnetic resonance analysis, and the spectrogram data obtained are as follows : 1 H NMR (300MHz, CDCl 3): δ=8.18(d, J=7.6Hz, 2H), 8.09(s, 1H), 7.36(d, J=7.6Hz, 2H), 7.12(s, 1H), 6.94-6.90(m, 3H) , 6.80(s, 1H), 6.78(s, 1H), 3.53-3.48(m, 1H), 3.11-3.06(m, 1H), 2.47-2.45(m, 2H), 2.44(s, 3H), 2.09 -1.95 (m, 2H) ppm. From the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com