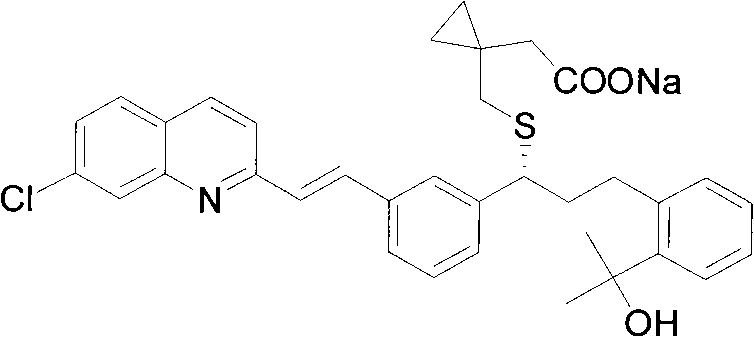
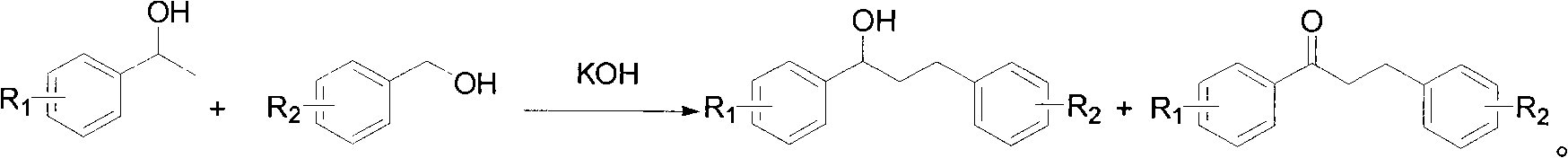
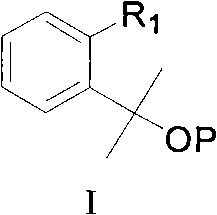
Montelukast sodium intermediate and method for synthesizing montelukast sodium thereof
A compound and mixture technology, applied in the field of montelukast sodium new intermediate, synthesis of montelukast sodium, can solve the problems of complex and harsh conditions, many by-products, unsuitable for large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: [2-[1-methyl-1-(tetrahydropyran-2-oxyl group) ethyl] phenyl]-methanol synthesis
[0098] Step 1: In an ice bath at -15°C, add 50 grams of phthalide and 200 milliliters of tetrahydrofuran to a 2000 milliliter three-necked round-bottomed flask, protect it with nitrogen, and then slowly add 400 milliliters of CH 3 The tetrahydrofuran solution of MgCl (2.0 mol / liter), dripped within 2 hours, then slowly warmed up to room temperature, and after 4 hours, the reaction solution was poured into a flask with 500 ml of saturated ammonium chloride ice-water solution, stirred, and dissolved in acetic acid Ethyl ester (4 × 200 ml) was extracted, the ethyl acetate layers were combined, washed with 10% brine (3 × 200 ml), dried by adding magnesium sulfate, the solvent was spun to dryness, and vacuum dried for 4 hours to obtain 57 g of white waxy solid , yield 92.0%. 1 H NMR (400MHz, CDCl 3 ): δ1.59 (s, 6H), 4.52 (s, 2H), 4.71 (s, 2H), 7.27-7.17 (m, 4H).
[0099] The seco...
Embodiment 2
[0102] Embodiment 2: the synthesis of [2-[1-methyl-1-(methoxy) ethyl] phenyl]-methanol
[0103] The first step: the same as the first step in embodiment 1.
[0104] The second step: dissolve 55 grams of the waxy solid obtained in the first step in 115 milliliters of dichloromethane, add 100 milliliters of triethylamine, 0.4 grams of 4-dimethylaminopyridine, and slowly drop into the dissolved 62.5 grams of tert-butyldimethylchlorosilane in 50 milliliters of dichloromethane were reacted for 4 hours. After the reaction was completed, 200 milliliters of saturated sodium bicarbonate solution was added, the layers were separated, and the water layer was washed with dichloromethane (2×150 milliliters) ), the dichloromethane layers were combined, washed with saturated brine (3×200 ml), dried by adding magnesium sulfate, and the solvent was spun to dryness to obtain an oil, which was directly used in the next step.
[0105] The third step: at room temperature, take part of 28 grams of...
Embodiment 3
[0107] Example 3: 1-[3-[2-(7-chloro-2-quinolyl)-vinyl]phenyl]-3-[2-(1-hydroxyl-1-methyl)ethylphenyl Synthesis of ]-propan-1-one (compound of formula III)
[0108] Take 3.09 grams of 1-[3-[2-(7-chloro-2-quinolyl)vinylphenyl]-ethanol, dissolve it in 20 ml of toluene, and add [2-[1-methyl-1-( Tetrahydropyran-2-oxygen) ethyl] phenyl]-methanol 3.0 grams, potassium hydroxide 1.2 grams, reflux for 6 hours, after reaction, add 100 milliliters of saturated NH4Cl solution, with ethyl acetate (3 * 40 ml), combined the ethyl acetate layers, washed with saturated brine, dried over magnesium sulfate, and evaporated to dryness to obtain an oil. Dissolve the oil in 20 ml of dichloromethane, add 4.2 g of Dess-Martin oxidant at room temperature, react for 4 hours, filter through diatomaceous earth, spin to dry the solvent, add 20 ml of methanol, add 0.75 g of p-tolylidine sulfonate Acetate, reacted at room temperature for 10 hours, evaporated methanol to dryness, added 30 ml of water, extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com