A kind of preparation method of royal jelly acid
A technology of royal jelly acid and tetraalkylammonium bromide, which is applied in the field of preparation of royal jelly acid, can solve the problems of unstable Grignard reagent, high requirements for reaction conditions, and high production cost, achieve high selectivity and yield, and be easy to industrialize The effect of easy production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
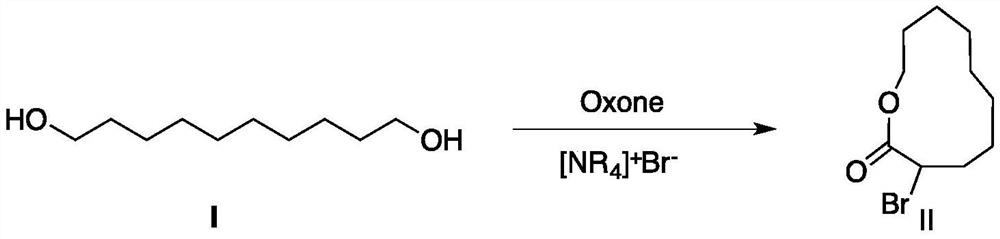
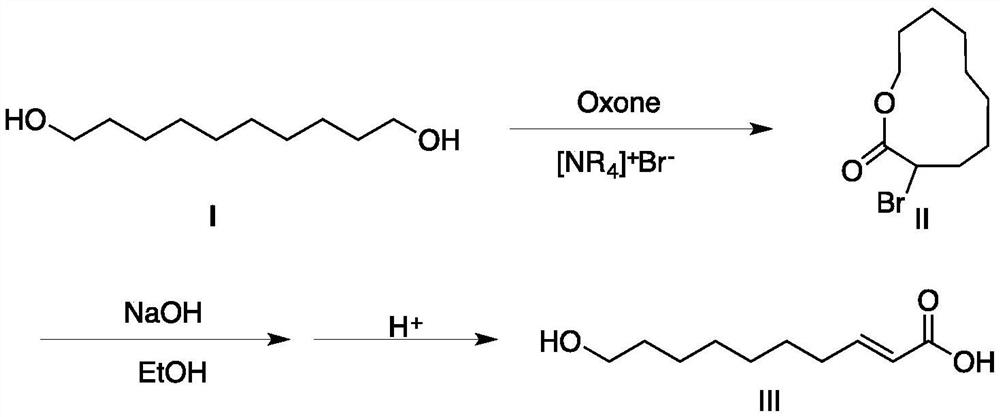
[0026] Add 17.4 grams (0.1mol) of 1,10-decanediol, 74.1 grams (0.23mol) of tetrabutylammonium bromide, 141.4 grams (0.23mol) of Oxone and 200 milliliters of water in a 500mL single-necked bottle, and the mixture is heated to 80 degree, and reacted at this temperature for 6h. The reaction was carried out by TLC (the developing solvent was petroleum ether / ethyl acetate (volume ratio 1:2), R f = 0.4). After the reaction was completed, the reaction solution was cooled to about 0°C, the precipitated product was filtered and washed with water, and after drying, 24.0 g of white product II was obtained, with a yield of 96.3%.
[0027] 1 HNMR (CDCl 3 , δ (ppm), TMS): δ1.25-1.30 (m, 10H), 1.55 (m, 2H), 1.99-2.25 (m, 2H), 3.62 (t, 2H), 4.08 (t, 2H).
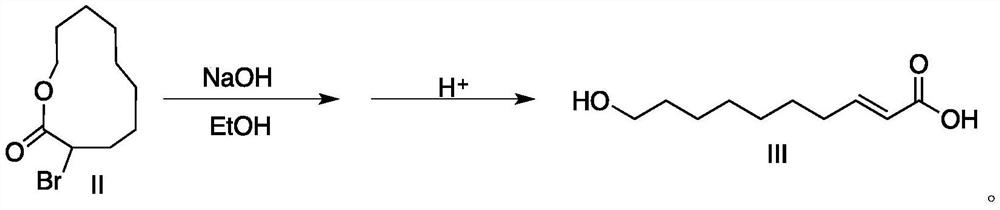
[0028] In a 250mL single-necked flask, add 12.5 grams (0.05mol) of the II compound prepared above, 40mL15wt% NaOH (0.15mol) ethanol solution, and stir at room temperature for 3h; Add concentrated hydrochloric acid drop by drop under ic...
Embodiment 2
[0031] Add 17.4 grams (0.1mol) of 1,10-decanediol, 35.4 grams (0.23mol) of tetramethylammonium bromide, 141.4 grams (0.23mol) of Oxone and 200 milliliters of water in a 500mL single-necked bottle, and heat the mixture to 80 degree, and reacted at this temperature for 7h. The reaction was carried out by TLC (the developing solvent was petroleum ether / ethyl acetate (volume ratio 1:2), R f = 0.4). After the reaction was completed, the reaction liquid was cooled to about 0°C, the precipitated product was filtered and washed with water, and after drying, 18.70 g of white product II was obtained, with a yield of 78.0%.
[0032] Add 12.5 grams (0.05mol) of compound II prepared above and 50mL of 15wt% NaOH (0.188mol) ethanol solution in a 250mL single-necked flask, stir and react at room temperature for 2.5h; Add concentrated hydrochloric acid drop by drop in an ice bath to adjust the pH of the solution to 3-4, put it in the refrigerator overnight to crystallize, filter to obtain the ...
Embodiment 3
[0034] Add 17.4 grams (0.1mol) of 1,10-decanediol, 151.6 grams (0.23mol) of tetra-n-decylammonium bromide, 141.4 grams (0.23mol) of Oxone and 200 milliliters of water in a 500mL single-necked bottle, and the mixture is heated to 80 degrees, and react at this temperature for 10h. The reaction was carried out by TLC detection (developing solvent was petroleum ether / ethyl acetate (volume ratio 1:2),
[0035] R f = 0.4). After the reaction was completed, the reaction solution was cooled to about 0°C, the precipitated product was filtered and washed with water, and after drying, 20.5 g of white product II was obtained, with a yield of 82.4%.
[0036] The steps of preparing royal jelly acid by hydrolysis, elimination and acidification of compound II are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com