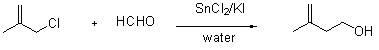Method for synthesizing 3-methyl-3-butene-1-ol
A synthesis method and technology of methacrylic acid, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of high cost and harsh process conditions, and achieve low production cost, low environmental requirements, The effect of simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 500 mL three-necked flask, add 90 g of 3-chloro-2-methylpropene, 30.6 g of paraformaldehyde, 248 g of stannous chloride dihydrate, 1.1 g of potassium iodide, and 180 g of water in sequence. Under the condition of a stirring speed of 30r / min, the reaction was stirred for 4.5h, and the reaction of 3-chloro-2-methylpropene was detected by gas chromatography to be complete. Stop the reaction, and extract with 600 mL of ethyl acetate three times. Combine the extracts, wash the extracts with 300 mL of saturated NaCl solution three times, let the layers rest, and separate the water layer. Dry the organic layer with 50 g of anhydrous magnesium sulfate. After drying for 0.5 h, use a neckless funnel to plug cotton to separate and remove the desiccant. The obtained organic solvent is collected as a transparent liquid at 72 °C under a vacuum of 0.1 atmosphere. 85g, proved by Bluker AVANCE / AV400 nuclear magnetic resonance instrument (Bruker, Germany), that the product is 3-met...
Embodiment 2
[0031] Into a 500 mL three-necked flask, 90 g of 3-chloro-2-methylpropene, 31.0 g of paraformaldehyde, 271 g of stannous chloride dihydrate, 2.1 g of potassium iodide, and 162 g of water were sequentially added. Under the condition of a stirring speed of 35 r / min, the reaction was stirred for 4 h. Stop the reaction, and extract with 600 mL of ethyl acetate three times. Combine the extracts, wash the extracts with 300 mL of saturated NaCl solution three times, let the layers rest, and separate the water layer. Dry the organic layer with 50 g of anhydrous magnesium sulfate. After drying for 0.5 h, use a neckless funnel to plug cotton to separate and remove the desiccant. The obtained organic solvent is collected as a transparent liquid at 72 °C under a vacuum of 0.1 atmosphere. 81 g, the liquid is 3-methyl-3-buten-1-ol. 1 HNMR (CDCl 3 ), δ: 1.75(s, 3H, -CH 3 ),2.27-2.30(t, J = 5.6Hz, 2H, -CH 2 -), 2.34(s, 1H, -OH), 3.68-3.72(t, J = 6.4Hz, 2H, -CH 2 -), 4.77(s, 1H), 4.84(s,...
Embodiment 3
[0033] In a 500 mL three-necked flask, 90 g of 3-chloro-2-methylpropene, 31.2 g of paraformaldehyde, 293 g of stannous chloride dihydrate, 1.7 g of potassium iodide, and 216 g of water were sequentially added. Under the condition of a stirring speed of 35 r / min, the reaction was stirred for 5 h. Stop the reaction, and extract with 600 mL of ethyl acetate three times. Combine the extracts, wash the extracts with 300 mL of saturated NaCl solution three times, let the layers rest, and separate the water layer. Dry the organic layer with 50 g of anhydrous magnesium sulfate. After drying for 0.5 h, use a neckless funnel to plug cotton to separate and remove the desiccant. The obtained organic solvent is collected as a transparent liquid at 72 °C under a vacuum of 0.1 atmosphere. 85 g, the product was 3-methyl-3-buten-1-ol. 1 HNMR (CDCl 3 ), δ: 1.75(s, 3H, -CH 3 ),2.27-2.30(t, J = 5.6Hz, 2H, -CH 2 -), 2.34(s, 1H, -OH), 3.68-3.72(t, J = 6.4Hz, 2H, -CH 2 -), 4.77(s, 1H), 4.84(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com