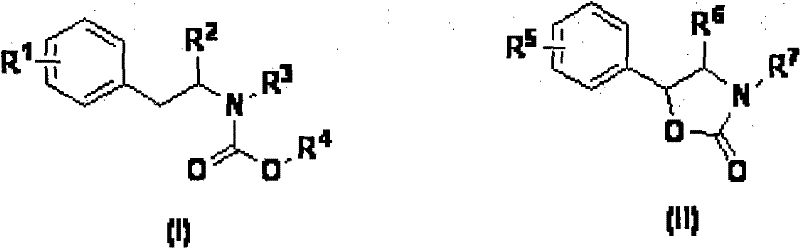
Synthesizing method of oxazolidine compound
A technology of oxazolidinone and synthesis method, applied in the field of synthesis of oxazolidinone compounds, can solve problems such as the influence of reaction speed, and achieve the effects of mild conditions, wide application range and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the phenylethylamine derivatives used in the present invention is as follows: react with commercially available p-methoxyphenethylamine or p-hydroxyphenylethylamine and benzaldehyde, and use tert-butoxycarbonyl chloride or It can be obtained after protection of benzyloxycarbonyl chloride.
[0028] The various organic oxidizing agents used in the present invention are all commercially available and used directly without purification.
[0029] The nuclear magnetic resonance instrument model used in the present invention is: Japan Electronics (JOEL) JNM-ECX400P.
Embodiment 1
[0031]
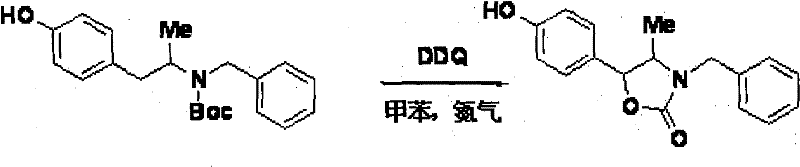
[0032] Under the protection of nitrogen at room temperature, add 240 mg of raw materials, 15 mL of toluene, and 175 mg of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) to a 100 mL single-necked bottle to prepare a reaction solution, stir and heat to 86 °C , track the reaction with thin layer chromatography (TLC) until most of the raw materials disappear, stop heating and cool to room temperature, the reaction solution is washed with saturated sodium carbonate solution, extracted with ethyl acetate, the organic phase is dried with anhydrous sodium sulfate, filtered, and distilled under reduced pressure After the solvent was removed, 124 mg of a red solid was obtained by separation on a silica gel column with a yield of 60%. It was identified as the target compound by NMR, and the trans compound was the main one.
[0033] 1 H NMR (400MHz, CDCl 3 )δ7.29-7.38(m, 5H), 7.09(d, J=8.0Hz, 2H), 6.82(d, J=8.0Hz, 2H), 6.08(s, 1H), 4.86(d, J=7.8 Hz, 1H), 4.81(d, J=15.1Hz, 1H)...
Embodiment 2
[0035]
[0036] Under nitrogen protection at room temperature, 170 mg of raw materials, 113 mg of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ), 6 mL of 1,4-dioxane and 0.6 mL of water were added to a 25 mL three-neck flask to prepare into a reaction solution. Heated to reflux, the reaction solution was a brown solution. Use TLC to track the reaction to the end, cool to room temperature, add 10mL saturated sodium bicarbonate solution to the reaction solution, extract with 20mL ethyl acetate, combine the organic phases, wash the organic phase twice with 20mL water, dry over anhydrous sodium sulfate, reduce After the ethyl acetate was evaporated under pressure, it was separated and purified by column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain 116 mg of a colorless oily compound with a yield of 79%, which was identified as the target compound by NMR.
[0037] 1 H NMR (400MHz, CDCl 3 )δ7.22-7.39(m, 7H), 6.88(d, J=8.2Hz, 2H), 5.42(t, J=8.2Hz, 1H), 4.54(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com