Preparation and application of recombinant consensus interferon mutant polyethylene glycol conjugate
A technology of polyethylene glycol and polyethylene glycol propionaldehyde, which is applied to the preparation methods of interferon and peptides, and medical preparations of non-active ingredients, etc., which can solve the problems of short half-life in vivo, low biological activity, and strong immunogenicity and other issues to achieve the effect of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
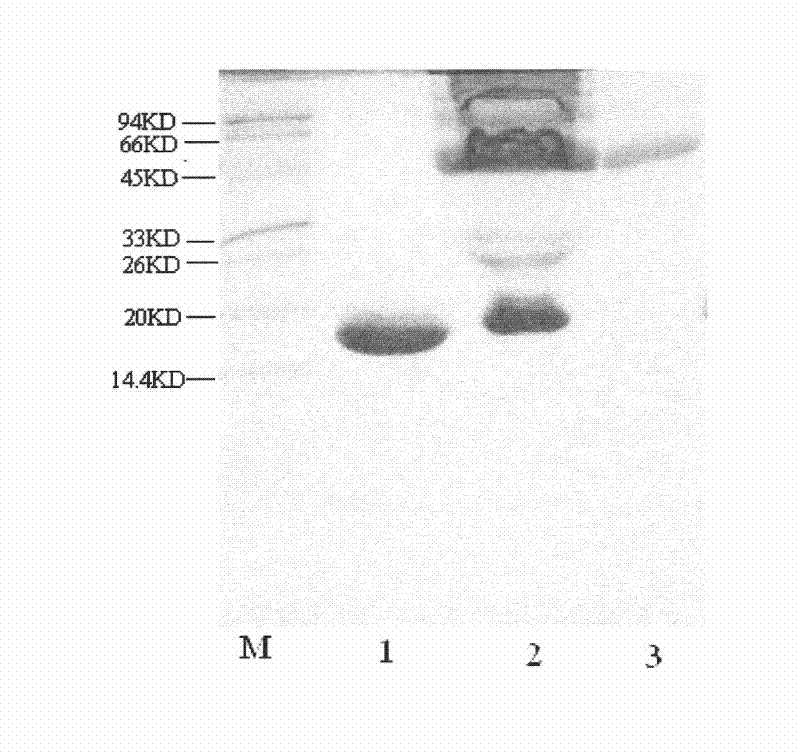
[0063] Example 1, Modification conditions of recombinant integrated interferon variant PEGylated conjugate (PEG20-IFN-SA)
[0064] IFN-SA solution (concentration: 10mg / mL, buffer: 100mM PB, pH7.0) was prepared by Chongqing Fujin Biopharmaceutical Co., Ltd., mPEG-ButyrALD-20KD (purity>95%, relative molecular mass: 20×10 3 ) was purchased from Beijing Jiankai Technology Co., Ltd., sodium cyanoborohydride (CH3BrNa) solution was purchased from Sigma Company, and SDS-PAGE related reagents and solutions and various analytical grades were all products of Shanghai Sangong. Constant temperature magnetic stirrer (Jiangsu Zhongda Instrument Factory), electrophoresis instrument, SP Sepharose F.F., Superdex75 molecular sieve, AKTA explore chromatography system, and SDS-PAGE electrophoresis system are all products of Pharmacia.
[0065] 1. The influence of reaction pH value on the modified product
[0066] The IFN-SA solution was prepared with 100mmol / L PB (pH4.0, pH5.0 and pH5.5) buffer s...
example 2
[0076] Example 2, Separation and Purification of Recombinant Integrated Interferon Variant PEGylated Conjugate (PEG20-IFN-SA)
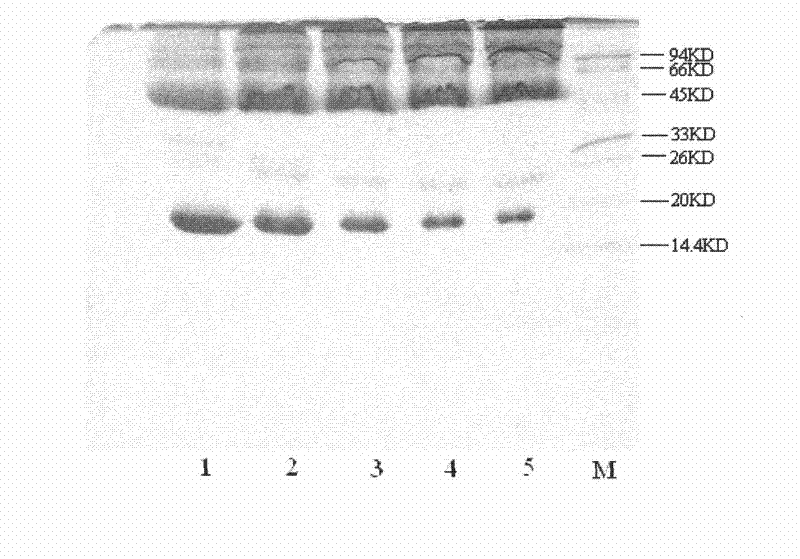
[0077] The modified sample PEG-IFN-SA was dialyzed overnight with 5×DDW, and the dialyzed sample was applied to SP Sepharose F.F chromatographic column. The SP Sepharose F.F chromatography column was equilibrated with BufferA (10mM acetic acid-sodium acetate pH5.5) and loaded on the sample, respectively, with elution 1 (10mM acetic acid-sodium acetate 0.15M NaCl pH5.5), elution 2 (10mM acetic acid -sodium acetate 0.2M NaCl pH5.5), elution 3 (10mM acetic acid-sodium acetate 0.25M NaCl pH5.5), elution 4 (10mM acetic acid-sodium acetate 0.35M NaCl pH5.5), elution 5 (10mM Acetic acid-sodium acetate (0.45MNaCl pH5.5) was used for elution, the breakthrough peak and each elution peak were collected respectively, and samples were taken for SDS-PAGE electrophoresis analysis. Collect the PEG-IFN-SA sample purified by SPSepharose F.F chromatography, and put it ...
example 3、10
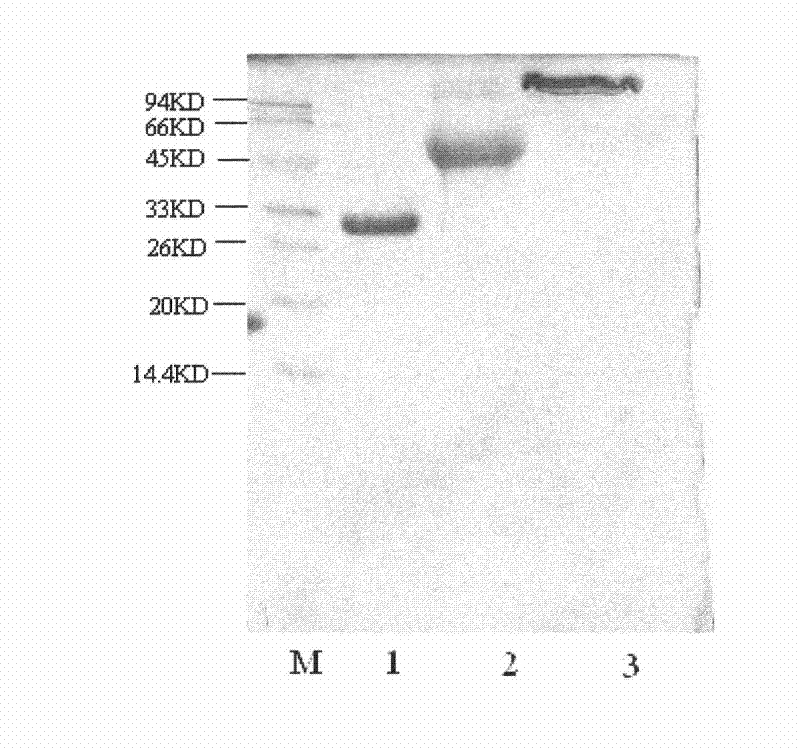
[0079] Example 3, 10KD and 40 polyethylene glycol modified recombinant integrated interferon variants
[0080] Select respectively the monomethoxypolyethylene glycol butyraldehyde of 10KD and the branched chain monomethoxypolyethylene glycol propionaldehyde of 40KD (both are the products of Beijing Jiankai Biological Company) according to the method of example 1 and example 2 to IFN -SA was modified at the N-terminal single site specificity, and PEG10-IFN-SA and PEG40-IFN-SA were obtained after separation and purification (see image 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com