Bi-component drug micromolecule hydrogel based on hexadecadrol and taxol and preparation method thereof
A technology of dexamethasone and small molecule water, applied in the direction of drug combination, pharmaceutical formula, chemical instrument and method, etc., to save the life of patients, prevent recurrence and metastasis, simple synthesis steps and purification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
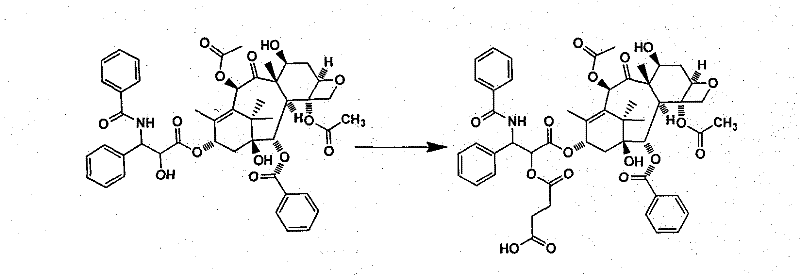
[0014] (1) Synthesis of Taxol-SA and Taxol-SA-NHS (compound 3 and compound 3a), the synthesis steps are shown in the appendix figure 1
[0015] 0.50 g (0.59 mmol) of paclitaxel was dissolved in 12 mL of pyridine, then 0.90 g (0.76 mmol) of succinic anhydride was added, and stirred at room temperature for 3 hours. The solution was removed by a rotary evaporator, then 20 mL of double-distilled water was added, stirred at room temperature for 20 minutes, then filtered, the precipitate was dissolved in acetone, and then water was added for crystallization, left to stand for 4 overnight, and the crystals were collected to obtain the product (yield 90%). (2) Synthesis of Dex-SA (Compound 4)
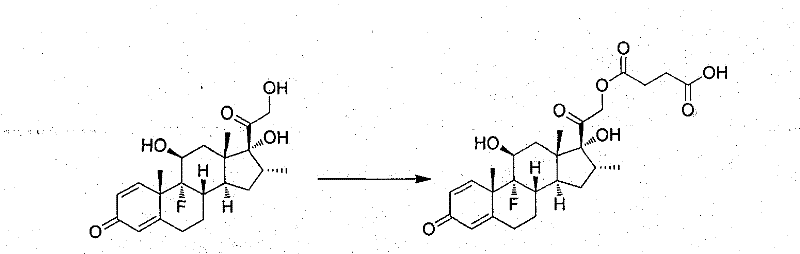
[0016] In the same reaction as above, weigh 1 g of dexamethasone and dissolve it in 20 mL of pyridine, add 3 equivalents of succinic anhydride at the same time, then add 0.1 equivalent of 4-dimethylaminopyridine, stir at room temperature for 24 hours, and remove the solution with a rotary evap...
Embodiment 2
[0019] Embodiment 2: the synthesis of compound Dex-FFFKESSEE and Dex-KESSEE
[0020] It was synthesized by standard Fmoc solid-phase synthesis method, and the amino acids used were all amino acids protected by Fmoc at the amino terminal, and the compound 4 added at the end can be regarded as an amino acid, and the way of connecting the peptide chain is the same as that of amino acids. The specific synthesis steps are as follows: (taking Dex-FFFKESSEE as an example)
[0021] ① Weigh 1mmol of resin, add it to a solid-phase synthesizer, add an appropriate amount of DCM to immerse the resin, and expand it for 5min under the blowing action of nitrogen; remove the solvent, add 1mmol of the first amino acid (glutamic acid protected by Fmoc in this experiment, and at the same time The side chain is also protected by OtBu) in DCM solution, add 2mmol DIEA, react for 2h;
[0022] ②Washing: wash with DCM, wash 5 times, each time for 1min;
[0023] ③ Sealing: react with a solution with a...
Embodiment 3
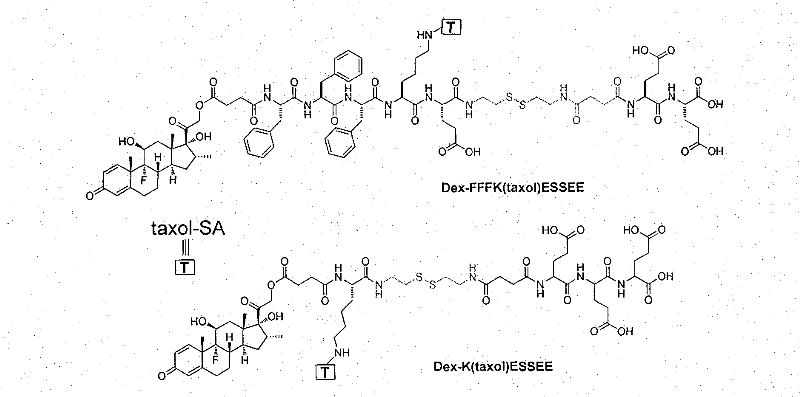
[0029] Example 3: Synthesis of colloidal precursor molecules Dex-FFFK (taxol-SA) ESSEE and Dex-K (taxol-SA) ESSEE (taking Dex-FFFK (taxol-SA) ES SEE as an example)
[0030] Dissolve 60mg (0.06mmol) 3a in 3mL dimethylformamide solvent, add 50μl DIEA at the same time, then add 121mg (0.07mmol) Dex-FFFKE-ss-EE to react overnight at room temperature, and use high performance liquid phase to purify the compound to obtain Dex -FFFK(taxol-SA)ESSEE, yield 87.0%. 1 H NMR (400MHz, DMSO-d6) δ8.12-8.19 (m, 4H), 8.02-8.09 (m, 4H), 7.97-7.99 (m, 3H), 7.86 (d, J=7.13, 1H), 7.72 -7.76(m, 1H), 7.64-7.67(m, 2H), 7.54-7.58(m, 1H), 7.48-7.50(m, 2H), 7.44-7.47(m, 4H), 7.28-7.31(m, 1H), 7.24-7.26(m, 4H), 7.16-7.20(m, 12H), 6.30(s, 1H), 6.21-6.24(m, 1H), 6.01(s, 1H), 5.80-5.85(m, 1H), 5.51-5.55(m, 1H), 5.40-5.42(m, 2H), 5.33-5.35(m, 1H), 5.17(s, 1H), 4.99-5.04(m, 1H), 4.90-4.95( m, 2H), 4.73-4.78(m, 1H), 4.64(s, 1H), 4.59-4.61(m, 1H), 4.44-4.50(m, 2H), 4.23-4.28(m, 3H), 4.09- 4.17(m, 4H), 3.98-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com