New method for synthesizing tapentadol hydrochloride and analogue of tapentadol hydrochloride
A technology of hydrochloric acid and compounds, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as increasing the difficulty of industrial production, difficult practical operation, and unfavorable industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
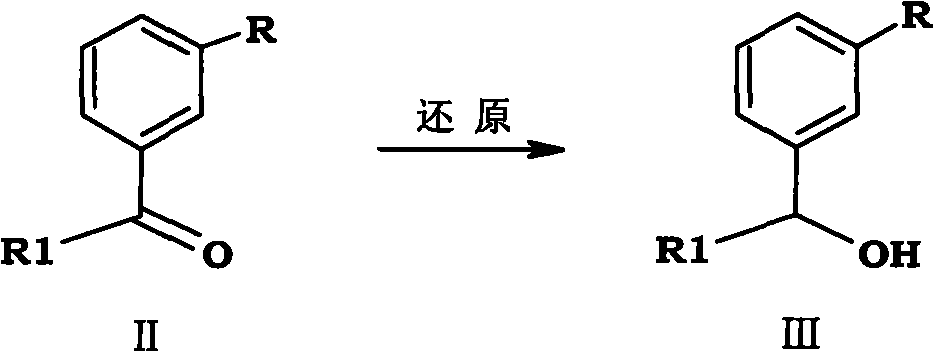
[0091] Example 1 Preparation of 1-(3-methoxyphenyl)-propan-1-ol
[0092] Under ice-bath conditions, add 1.5L of methanol and 700g (4.26mol) of 1-(3-methoxyphenyl)propan-1-one into the reaction flask, stir and inject N 2 , when the system drops to about 5°C, add 201.7g (5.34mol) of 96% sodium borohydride in 4 times, continue the heat preservation reaction for 30 minutes after the addition, TLC traces the completion of the reaction, distills off the solvent under reduced pressure, and pours the reactant into 2L of water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 700 g of 1-(3-methoxyphenyl)-propan-1-ol with a yield of 98.9%.
[0093] Molecular formula: C 10 h 14 o 2 , molecular weight: 166.22, MS (m / z): 167.21 (M + +H).
[0094] 1 HNMR (CDCl 3 , 400MH Z )δ: 7.12(t, 1H, J=8.0Hz, Ar-H), 6.81(d, 1H, J=8.0Hz, Ar-H), 6.67(m, 2H, Ar-H), 4.62(t, 1H, J=5.2Hz, CH-O), 3.75(s, 3H, -OCH 3 ), 1.81 (m, 2H,...
Embodiment 2
[0095] Example 2 Preparation of 1-(3-nitrophenyl)-propan-1-ol
[0096] Substitute 1-(3-methoxyphenyl) propan-1-one in Example 1 with 1-(3-nitrophenyl) propan-1-one to obtain compound 1-(3-nitrophenyl)- Propan-1-ol. The yield is 97.1%.
[0097] Molecular formula: C 9 h 11 NO 3 , molecular weight: 181.2, MS (m / z): 182.18 (M + +H).
Embodiment 3
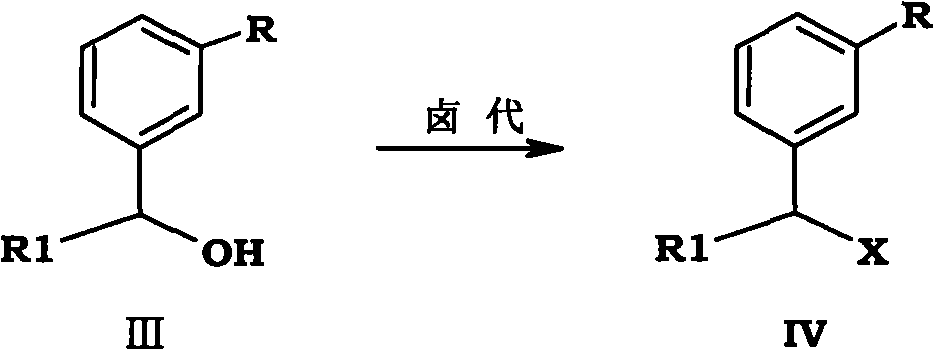
[0098]Example 3 Preparation of 3-(1-bromopropyl) anisole
[0099] in N 2 Under protection, 700g (4.21mol) of 3-(3-methoxyphenyl)-2-pentanol and 2L of dichloromethane were added to the reaction flask, and when the temperature was cooled to about 5°C in an ice bath, 1.14Kg ( 4.21mol) PBr 3 , keep the temperature, continue to insulate and stir the reaction for 1h after the addition is completed, and follow the completion of the reaction by TLC. The reactant is poured into ice water, extracted with dichloromethane, the organic layer is washed with aqueous sodium bicarbonate, then washed with water, and dried over anhydrous magnesium sulfate. After concentration under reduced pressure, 920 g of 3-(1-bromopropyl) anisole was obtained, with a yield of 95.4%.
[0100] Molecular formula: C 10 h 13 BrO, molecular weight: 229.11, MS (m / z): 230.10 (M + +H).
[0101] 1 HNMR (CDCl 3 , 400MH Z )δ: 7.27(t, 1H, J=8.4Hz,, Ar-H), 6.68(m, 2H, Ar-H), 6.51(s, 1H, Ar-H), 4.67(t, 1H, J= 4.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com