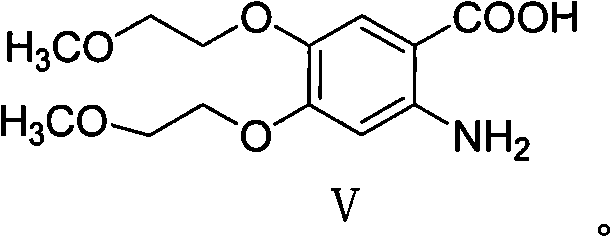
Synthesis intermediate of erlotinib and preparation method thereof
A form and compound technology, applied in the field of pharmaceuticals, can solve problems such as high cost of raw materials, large pollution, troublesome product purification and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Synthesis of 4-bis-(2-methoxyethoxy)-benzaldehyde (II)
[0051]
[0052] Put 25g of 3,4-dihydroxybenzaldehyde, 60ml of 2-bromoethyl methyl ether, 120ml of dimethylformamide (DMF) and 60g of potassium carbonate into a 500ml reaction flask, and react at 100°C 3-4 hours. After the completion of the reaction as detected by TLC, the DMF was evaporated under reduced pressure, extracted with dichloromethane, washed with water, dried over anhydrous sodium sulfate, filtered, and dichloromethane was evaporated to obtain 45 g of brown oil with a yield of 97.8%.
[0053] 1 H NMR (400MHz, CDCl 3 )δ: 9.84(s, 1H), 7.43~7.46(m, 2H), 7.00(d, J=8.1Hz, 1H), 4.21~4.25(m, 4H), 3.79~3.83(m, 4H), 3.47 (s, 6H)
Embodiment 2
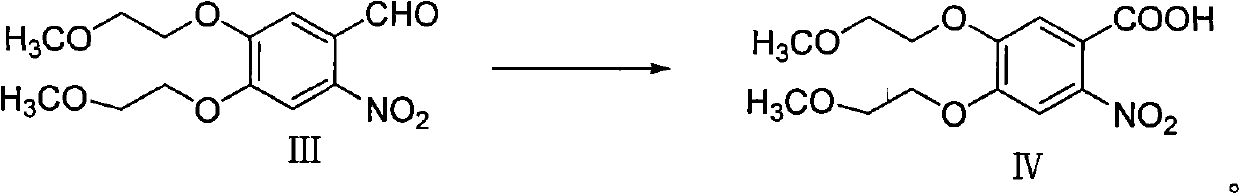
[0054] Example 2 Synthesis of 2-nitro-4,5-bis-(2-methoxyethoxy)-benzaldehyde (Ⅲ)
[0055]
[0056] 45g of compound II was dissolved in 250ml of glacial acetic acid, the temperature was controlled at 0-10°C, and 94ml of nitric acid was slowly added dropwise at this temperature. After the dropwise addition was completed, it was raised to room temperature and stirred for 12 hours. The next day, the reaction solution was poured into ice water, and ammonia water was slowly added dropwise to adjust the pH of the system to 7-8, filtered, and dried to obtain 38 g of a light yellow solid. The yield was 71.8%.
[0057] 1 HNMR (CDCl 3 , 400MHz) δ: 10.43(s, 1H), 7.67(s, 1H), 7.43(s, 1H), 4.29~4.32(m, 4H), 3.82~3.85(m, 4H), 3.46(s, 6H)
Embodiment 3
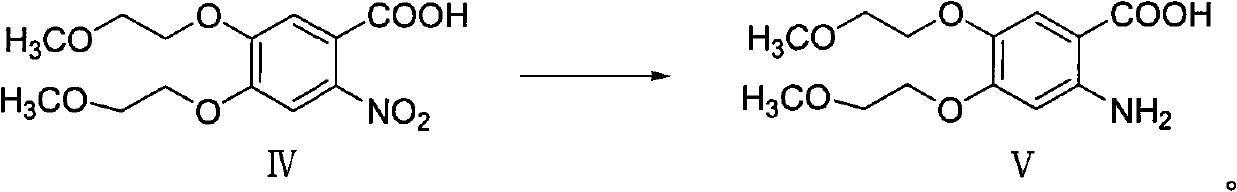
[0058] Example 3 Synthesis of 2-nitro-4,5-bis(2-methoxyethoxy)-benzoic acid (Ⅳ)
[0059]
[0060] Dissolve 30g of compound III in 180ml of methanol, add 0.5ml of 30% methanolic sodium hydroxide solution, heat to 45°C, slowly add 60ml of 35% hydrogen peroxide dropwise, and complete the dropwise addition in 3-4 hours, keeping the pH of the solution at 10.5- 11.5, TLC detected that the reaction was complete, poured the reaction solution into ice water, extracted three times with dichloromethane, combined the dichloromethane layers, dried, and concentrated to obtain 24 g of a brownish-red oil with a yield of 75.9%.
[0061] 1 HNMR (MDSO-d, 400MHz) δ: 13.60(brs, 1H), 7.59(s, 1H), 7.31(s, 1H), 4.24~4.27(m, 4H), 3.67-3~69(m, 4H) , 3.31(s, 6H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com