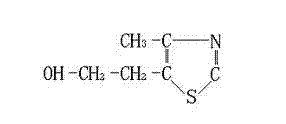
Synthesis method for 4-methyl-5-(2-ethoxyl)-thiazole
A synthesis method and hydroxyethyl technology, applied in the direction of organic chemistry, etc., can solve the problems of many reaction steps, large amount of three wastes, low yield, etc., and achieve the effect of reducing reaction steps, low toxicity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Ammonification:
[0039] Introduce ammonia into carbon disulfide to obtain ammonium salt; carbon disulfide: ammonia = 1:1.2, molar ratio; reaction temperature: 15°C;
[0040] 2) Chlorination:
[0041] In a 500ml four-neck flask equipped with a stirrer and a temperature measuring device, add 0.2mol of α-acetyl-γ-butyrolactone, add CaCl 2 and NaHPO 4 125ml of mixed solution; NaHPO 4 The concentration is 0.5mol / L; CaCl 2 : NaHPO 4 =1:1, molar ratio; then pass chlorine gas under the irradiation of ultraviolet light, hydrolyze to get 3-chloroacetylpropanol; 2-acetylbutyrolactone: chlorine gas=1:1, molar ratio; reaction time 1.5 hours, The reaction temperature is 15°C; more preferably, ultraviolet light with a wavelength of 200nm is used;
[0042] 3) Condensation:
[0043] The 3-chloroacetylpropanol obtained in step 2) is condensed with the ammonium salt obtained in step 1) to obtain a condensate; the ratio of 3-chloroacetylpropanol to the ammonium salt obtained in ...
Embodiment 2
[0049] 1) Ammonification:
[0050] Introduce ammonia into carbon disulfide to obtain ammonium salt; carbon disulfide: ammonia = 1:1.1, molar ratio; reaction temperature: 10°C;
[0051] 2) Chlorination:
[0052] In a 500ml four-neck flask equipped with a stirrer and a temperature measuring device, add 0.2mol of α-acetyl-γ-butyrolactone, add CaCl 2 and NaHPO 4 125ml of mixed solution; NaHPO 4 The concentration is 1.2mol / L; CaCl 2 : NaHPO 4 =1:1, molar ratio; then pass chlorine gas under the irradiation of ultraviolet light, hydrolyze to get 3-chloroacetylpropanol; 2-acetylbutyrolactone: chlorine gas=1:0.8, molar ratio; reaction time is 2 hours, The reaction temperature is 10°C; use ultraviolet light with a wavelength of 250nm;
[0053] 3) Condensation:
[0054] The 3-chloroacetylpropanol obtained in step 2) is condensed with the ammonium salt obtained in step 1) to obtain a condensate; the ratio of 3-chloroacetylpropanol to the ammonium salt obtained in step 1) is 1: 4, m...
Embodiment 3
[0059] 1) Ammonification:
[0060] Introduce ammonia into carbon disulfide to obtain ammonium salt; carbon disulfide: ammonia = 1: 1.5, molar ratio; reaction temperature: 20°C;
[0061] 2) Chlorination:
[0062] In a 500ml four-neck flask equipped with a stirrer and a temperature measuring device, add 0.2mol of α-acetyl-γ-butyrolactone, add CaCl 2 and NaHPO 4 125ml of mixed solution; NaHPO 4 The concentration is 1.0mol / L; CaCl 2 : NaHPO 4 =1:0.9, molar ratio; then pass chlorine gas under the irradiation of ultraviolet light, hydrolyze to get 3-chloroacetylpropanol; 2-acetylbutyrolactone: chlorine gas=1:0.8, molar ratio; reaction time 1.5 hours, The reaction temperature is 20°C; more preferably, ultraviolet light with a wavelength of 300nm is used;
[0063] 3) Condensation:
[0064] The 3-chloroacetylpropanol obtained in step 2) is condensed with the ammonium salt obtained in step 1) to obtain a condensate; the ratio of 3-chloroacetylpropanol to the ammonium salt obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com