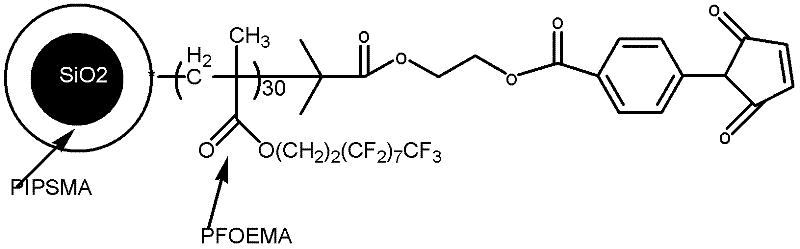
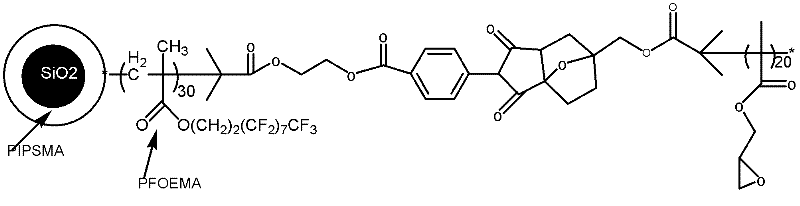
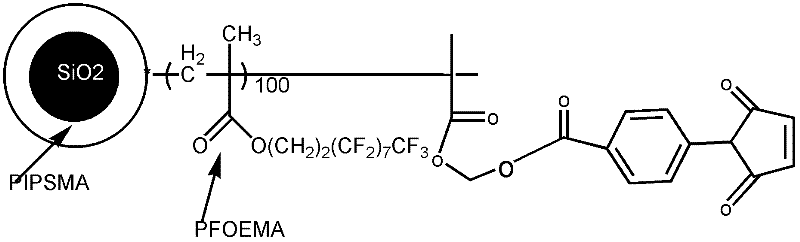
Fluorine-containing nanoparticles with high dispersibility and bonding property and superamphiphobic surface
A nano-microsphere, high-dispersion technology, applied in the direction of coating, etc., can solve the problems of expensive, toxic, unfavorable construction and environmental protection of fluorine-containing solvents, and achieve excellent hydrophobicity and oil repellency, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The end group is the preparation method of the fluoropolymer of compound D, comprises the following steps:
[0072] (1) Synthesis of Hydroxyl ATRP Initiator (HEBI)
[0073] Add 4ml of triethylamine to 25g of ethylene glycol (furfuryl alcohol), place it in an ice-water bath, slowly drop in 2.5ml of 2-bromoisobutyryl bromide, naturally warm up to room temperature and react for 4h, and then use saturated sodium bicarbonate solution to It was washed 3 times, then washed to neutrality with pure water, then dried with anhydrous magnesium sulfate, removed dichloromethane to obtain a viscous liquid substance, and then distilled under reduced pressure to obtain the ATRP initiator (HEBI) with hydroxyl ,
[0074] Nuclear magnetic spectrum analysis is: 1H NMR (CDCl 3 , δ, ppm): 4.35(q, 1H, J=6.89Hz), 4.19(t, 2H, J=4.75Hz), 3.75(t, 2H, J=4.75Hz), 3.00(s, 1H, OH) , 1.75 (d, 3H, J=6.96Hz).13C NMR (CDCl 3 , δ): 170.53, 67.18, 60.35, 39.93, 21.50. Therefore, it can be deduced that i...
Embodiment 2
[0085] The end group is the preparation method of the fluoropolymer of compound D, comprises the following steps:
[0086] (1) Synthesis of maleimido methacrylate
[0087] Dissolve 1.25g of ρ-CPMIC in 50ml of anhydrous dichloromethane, add 2.1ml of triethylamine, and slowly drop into 1.21ml of ATRP initiator hydroxyethyl methacrylate with hydroxyl groups under ice-water bath conditions. Naturally warm up to room temperature and react for 10 h, then wash it with saturated sodium bicarbonate solution 3 times, then wash with pure water until neutral, then dry with anhydrous magnesium sulfate, remove dichloromethane, and obtain viscous liquid material, and then distilled under reduced pressure to obtain an acrylic monomer with a maleic anhydride functional group, that is, maleamidomethacrylate (ρ-CPMIC-HEMA).
[0088] The spectral analysis of the product is as follows: 1H-MR (CDCl3 as solvent): 6.94 (hydrogen on maleimide, 2H), 7.85, 7.95 (hydrogen on phenyl, 4H), 1.93 (CH3, 3H);...
Embodiment 3
[0095] End group is the preparation method of the epoxy resin type polymer of compound A, comprises the following steps:
[0096] (1) Synthesis of furan ring initiator (furyl bromoisobutyrate)
[0097] Disperse 1.5g of furyl alcohol (furfuryl alcohol) in 30ml of anhydrous dichloromethane, add 4ml of triethylamine, slowly drop in 2ml of 2-bromoisobutyryl bromide under ice-water bath conditions, naturally warm up to room temperature and react for 4h, then Then wash it 3 times with saturated sodium bicarbonate solution, then wash it with pure water to neutrality, then dry it with anhydrous magnesium sulfate, remove dichloromethane, obtain a viscous liquid substance, and then distill under reduced pressure to obtain the furan ring Initiator.
[0098] The spectral analysis of the product is as follows: 1 H-NMR (CDCl 3 solvent): 7.30, 6.25, 6.19 (hydrogen on the furan ring, 3H), 5.19 (hydrogen on the methylene on furfuryl alcohol, 2H), 2.02 (hydrogen on the dibromoisobutyryl brom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com