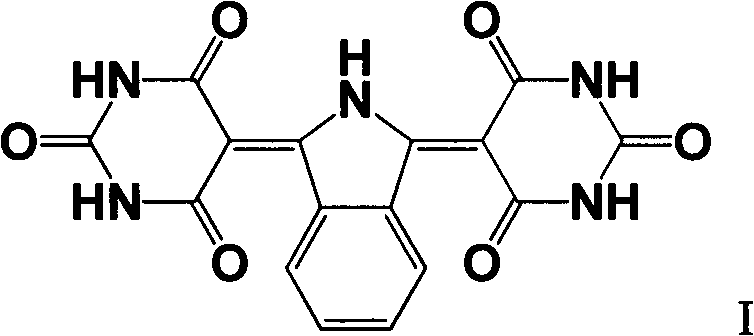
Method for preparing C.I. pigment yellow 139
A kind of pigment yellow, pigmentation technology, applied in the field of preparation of isoindoline pigments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The method for preparing C.I. Pigment Yellow 139 provided by the present invention specifically comprises the following steps:
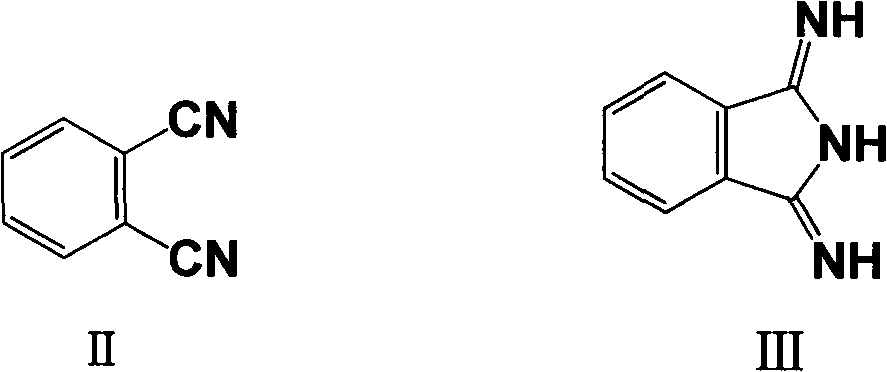
[0015] (1) Phthalonitrile (its structure is shown in formula II), catalyst and organic solvent are placed in the reactor, stirred, then ammonia water or ammonia gas is added, and the temperature is raised to 30 ° C ~ 60 ° C (preferably 40 ° C ~55°C), and kept in this state for 2 hours to 7 hours (preferably 3 hours to 4 hours), to obtain 1,3-diiminoisoindoline (its structure is shown in formula III);
[0016] (2) First react 1,3-diiminoisoindoline (its structure is shown in formula III) with a mixed solution (pH value of 2.5 to 5.0) composed of barbituric acid, acid and water for 1 hour ~ 2 hours, then heat up to the reflux state, and keep it in the reflux state for 2 hours to 5 hours, cool and filter to obtain a filter cake (crude pigment), and recover the mother liquor for recycling;
[0017] (3) Beat the filter cake obtained in step (2) in...
Embodiment 1
[0031] (1) Put 19.2g of phthalonitrile, 0.8g of triethylamine, and 150ml of methanol into a four-necked flask successively, start stirring, and slowly feed ammonia (measured by subtraction method, the total amount of feed is 3g), and at the same time Raise the temperature of the system. React at 40-50°C for 4 hours.
[0032] (2) In another flask, add 39.5g barbituric acid, 400ml water, start to stir, fully disperse, then add the mixture of 5.6g formic acid and sulfuric acid, then at room temperature by the resultant of step (1) gained in 1 The dropwise addition was completed within ~1.5 hours, and the temperature was raised to reflux for 2 hours. Then cool down and filter, the mother liquor is recycled, and the filter cake is set aside.
[0033] (3) Slurry the filter cake obtained in step (2) with water, add 0.4 g of di-sec-octyl sodium butenedioate, react under high pressure at 130 ° C for 3 hours, then filter, wash, and dry to obtain 52.54 g of pigment (abbreviated as pig...
Embodiment 2
[0035] (1) Put 19.2g of phthalonitrile, 1.2g of potassium carbonate, and 150ml of isopropanol into a four-necked flask in turn, and slowly add 12.6g of ammonia (25%) dropwise under stirring. After the dropwise addition, start Raise the temperature of the system. React at 40-50°C for 3-4 hours.
[0036] (2) in another flask, add 39.5g barbituric acid, 400ml water, start to stir, fully disperse, then add the mixture of 6.7g citric acid and hydrochloric acid, then at room temperature will be by the resultant of step (1) in 1 The dropwise addition was completed within ~1.5 hours, and the temperature was raised to reflux for 2 hours. Then cool down and filter, the mother liquor is recycled, and the filter cake is set aside.
[0037] (3) Beat the filter cake obtained by step (2) with water, add 0.54g of benzoic acid, react under high pressure at 105°C for 2 hours, then filter, wash, and dry to obtain 52.40g of pigment (abbreviated as pigment-2), The yield was 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com