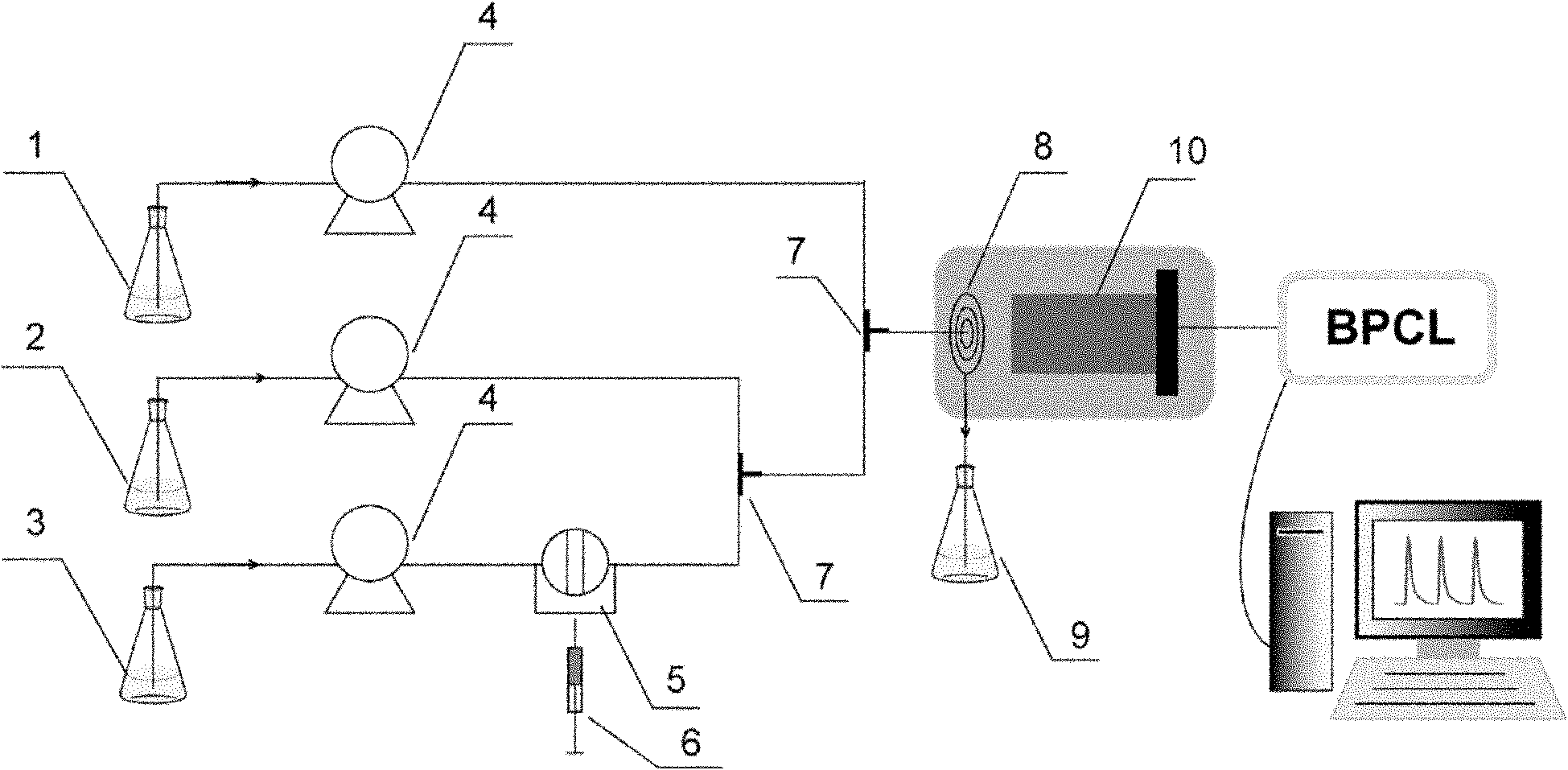
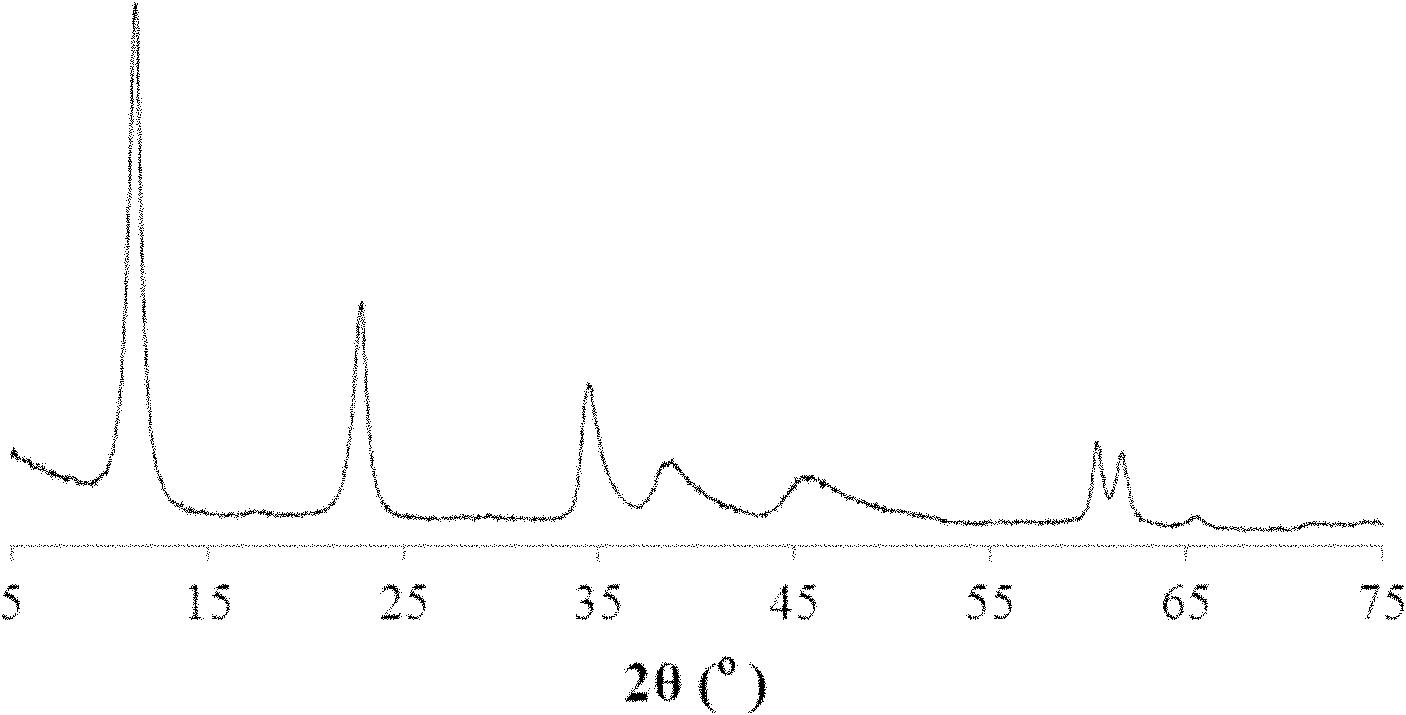
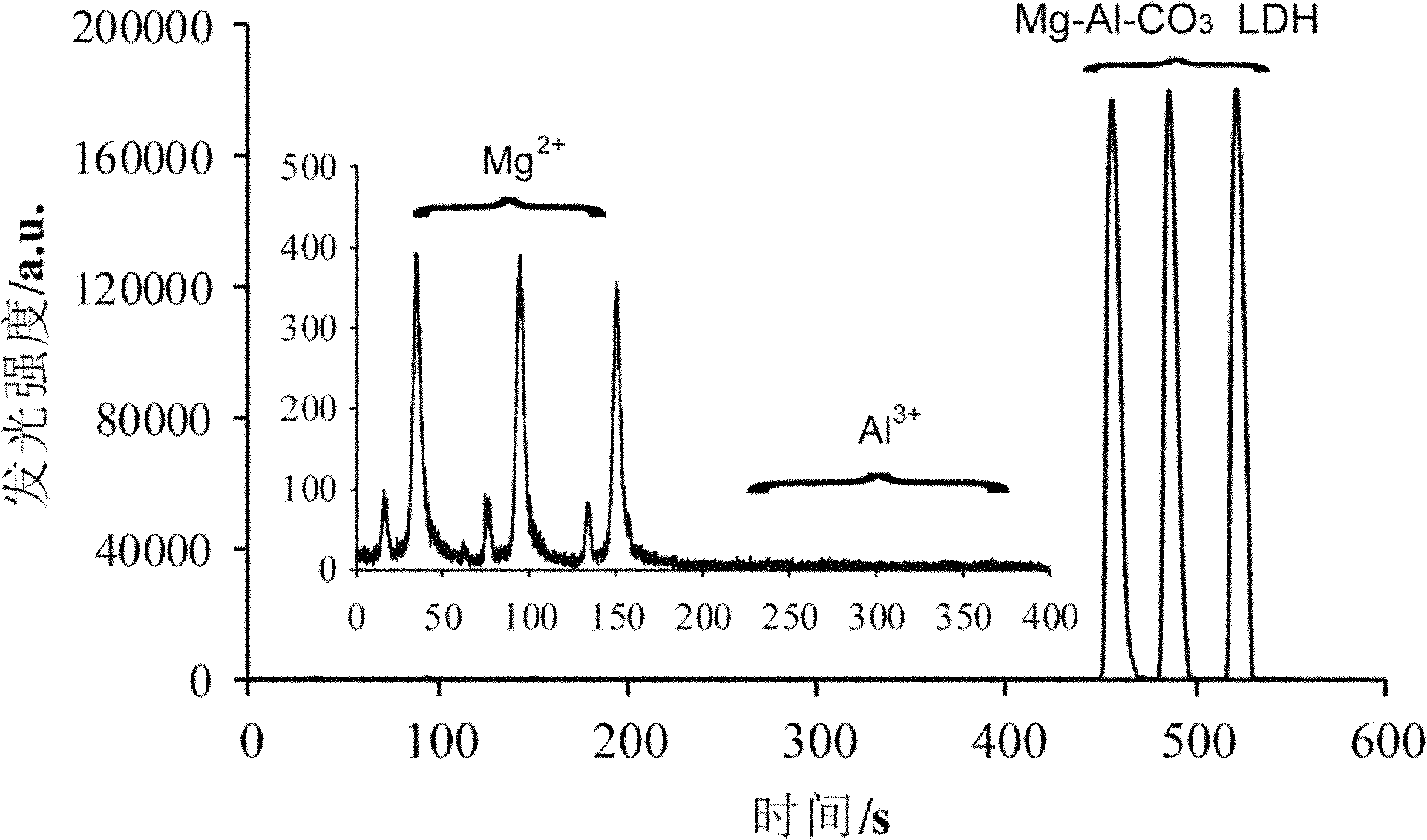
Analysis method for using magnesium-aluminium carbonate hydrotalcite to catalyze luminol-hydrogen peroxide chemiluminescence
A technology of hydrogen peroxide chemistry and analysis method is applied in the analysis field of catalyzing luminol-hydrogen peroxide chemiluminescence with magnesium-aluminum carbonate hydrotalcite, and can solve problems such as water pollution, pollution, environmental or ecosystem harm, and the like, Achieve the effect of easy preparation, high detection sensitivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Determination of hydrogen peroxide in tap water using magnesium aluminum carbonate hydrotalcite as a chemiluminescence catalyst.
[0020] A Prepare the solution to be tested and the running solution
[0021] The preparation method of the hydrotalcite of magnesium aluminum carbonate intercalation is: take by weighing 11.5385g Mg (NO 3 ) 2 ·6H 2 O and 5.6272gAl(NO 3 ) 3 9H 2 O, add 60mL ultrapure water to prepare salt solution; weigh 0.7949g Na 2 CO 3 With 4.8g NaOH, add 60mL ultrapure water to make lye. The above two solutions were dropped into the four-neck flask and stirred, the pH was controlled at 9.8-10.0, and the temperature was room temperature. After the dropwise addition, the four-necked bottle was placed in a water bath at 60°C for 24 hours for crystallization. The product was centrifuged and washed three times, dried in a vacuum oven at 70°C for 24 hours, and ground to powder. It is configured as 0.01-0.015g / mL magnesium aluminum carbonate...
Embodiment 2
[0031] Collect rainwater and measure it as soon as possible, and use the standard addition method to determine H in rainwater 2 o 2 concentration. Prepare the concentration of 2×10 respectively with rainwater -6 M, 4×10 -6 M, 6×10 -6 M, 8×10 -6 M, 10×10 -6 M and other hydrogen peroxide. Put into bottle No. 1.
[0032] Other steps are with embodiment 1. Measured H in rainwater 2 o 2 The concentration is 5.93×10 -6 M. The recovery rate was 102%.
[0033] Comparative Test:
[0034] With concentration being 0.375M magnesium nitrate solution, 0.125M aluminum nitrate solution to replace magnesium aluminum carbonate hydrotalcite in embodiment 1 respectively as catalyst, carry out the same operation result see image 3 . Depend on image 3 It can be seen that the chemiluminescence intensity of its magnesium nitrate solution and aluminum nitrate solution is far lower than the signal of magnesium aluminum carbonate hydrotalcite, indicating that the catalytic luminol-hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com