Multifunctional polyethylene glycol-dual vitamin E succinate derivative and application thereof in drug delivery
A technology of polyethylene glycol and succinate, applied in drug delivery, medical preparations of non-active ingredients, pharmaceutical formulations, etc., can solve the problems of high toxicity and low efficiency, and achieve easy operation and high encapsulation rate , the effect of easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
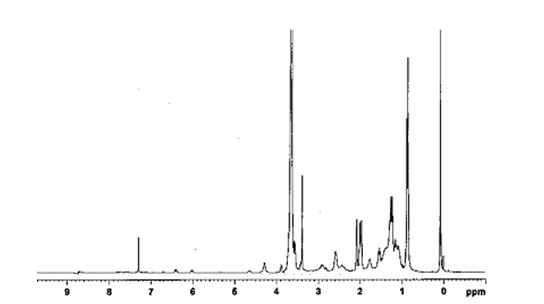
[0041] The preparation of embodiment 1 monomethoxypolyethylene glycol 2000-lysine-double vitamin E succinate block copolymer
[0042] (a) Dissolve 2.1 g vitamin E succinate in dichloromethane, add 1 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.6 g of 1-Hydroxybenzotriazole, magnetically stirred in an ice bath at 0°C for 1 h. 1.3 g of benzyloxylysine ester hydrochloride sulfonate was mixed with 3 mL of triethylamine, then added to the activation solution above, N 2 Under the protection of 30 ℃ magnetic stirring for 24 h. After the reaction, wash and dry. Purified to obtain benzyloxylysine di-vitamin E succinate (structural formula as formula Ⅰ).
[0043]
[0044] (b) Dissolve the product of step (a) in ethyl acetate, in palladium carbon (Pd / C) and H 2 Under the action of magnetic stirring at 30 °C for 6 h. Filtrate, collect the filtrate, and rotate to evaporate to obtain lysine di-tocopherol succinate (structural formula as formula II).
[...
Embodiment 2
[0049] Preparation of embodiment 2 polyethylene glycol 5000-lysine-double vitamin E succinate block copolymer
[0050] (a) Dissolve 2.1 g vitamin E succinate in dichloromethane, add 1 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.6 g of 1-Hydroxybenzotriazole, magnetically stirred in an ice bath at 0°C for 1 h. 1.3 g of benzyloxylysine ester hydrochloride sulfonate was mixed with 3 mL of triethylamine, then added to the activation solution above, N 2 Under the protection of 30 ℃ magnetic stirring for 24 h. After the reaction, wash and dry. Purified to obtain benzyloxylysine di-vitamin E succinate (structural formula as formula Ⅰ).
[0051]
[0052] (b) The product of step (a) was dissolved in ethyl acetate, and stirred magnetically at 30°C for 6 h under the action of palladium carbon and hydrogen. Filtrate, collect the filtrate, and rotate to evaporate to obtain lysine di-tocopherol succinate (structural formula as formula II).
[0053] ...
Embodiment 3
[0057] Embodiment 3 Preparation of monomethoxypolyethylene glycol amidation-lysine-double vitamin E succinate block copolymer
[0058] (a) Dissolve 2.1 g vitamin E succinate in dichloromethane, add 1 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and 0.6 g of 1-hydroxybenzotriazole (HOBT) was magnetically stirred in an ice bath at 0 °C for 1 h. 1.3 g of benzyloxylysine ester hydrochloride sulfonate was mixed with 3 mL of triethylamine, then added to the activation solution above, N 2 Under the protection of 30 ℃ magnetic stirring for 24 h. After the reaction, wash and dry. Purified to obtain benzyloxylysine di-vitamin E succinate (structural formula as formula Ⅰ).
[0059]
[0060] (b) Dissolve the product of step (a) in ethyl acetate, in palladium carbon (Pd / C) and H 2 Under the action of magnetic stirring at 30 °C for 6 h. Filtrate, collect the filtrate, and rotate to evaporate to obtain lysine di-tocopherol succinate (structural formula ...
PUM
| Property | Measurement | Unit |
|---|---|---|
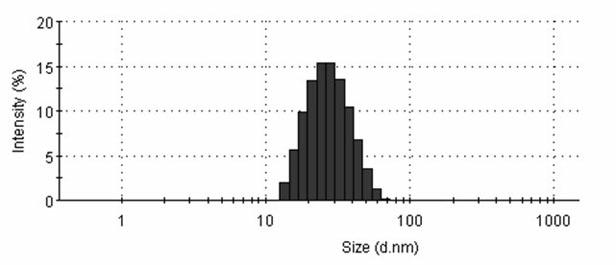
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com