Method of using macroporous strongly acidic type cation exchange resin to catalytically synthesize ethyl palmitate
A technology of strong acidic cations and ethyl palmitate, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., to achieve the effects of mild reaction conditions, reduced production costs, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
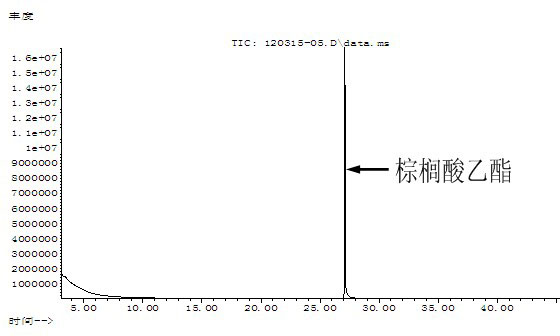
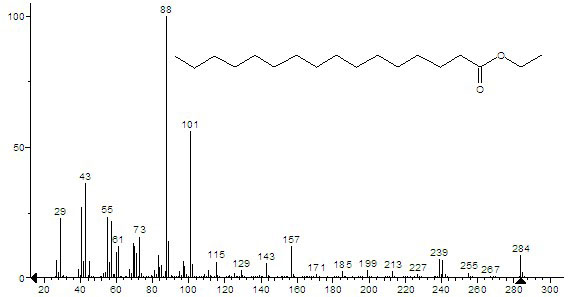
[0018] Example 1 : In a 100 ml round bottom flask, add 10.26 g (40 mmol) palmitic acid, 3.50 ml (60 mmol) absolute ethanol, 2.17 ml (20 mmol) cyclohexane, 5.13 g NKC-9 macroporous dry hydrogen catalytic resin , heated to reflux at 65°C under agitation, and water was continuously released from the water separator; the reaction was stopped when no more water was separated; the reaction solution was filtered to separate the resin, and the resin was washed with cyclohexane (10 ml for 2 times), separate and recover the resin, the washing liquid and the filtrate are combined to be separated; the reaction liquid is distilled at 80°C~90°C under normal pressure, and excess ethanol and cyclohexane are distilled off (ethanol and cyclohexane can be further recycled and reused), The resulting reaction solution was then distilled under reduced pressure at 65°C-67°C and a pressure of 1.33 kPa-1.35 kPa to collect fractions with a yield of 92%; the fractions were identified as ethyl palmitate...
Embodiment 2
[0019] Example 2 : In a 100 ml round bottom flask, add 10.26 g (40 mmol) palmitic acid, 4.67 ml (80 mmol) absolute ethanol, 2.17 ml (20 mmol) cyclohexane, 5.13 g NKC-9 macroporous dry hydrogen catalytic resin , heated to reflux at 70°C under agitation, and the water separator continuously released water, and stopped the reaction when no more water was separated; the reaction solution was filtered to separate the resin, and the resin was washed with cyclohexane (10 ml for 2 times), separate and recover the resin, the washing liquid and the filtrate are combined to be separated; the reaction liquid is distilled at 80°C~90°C under normal pressure, and excess ethanol and cyclohexane are distilled off (ethanol and cyclohexane can be further recycled and reused), The resulting reaction solution was then distilled under reduced pressure at 65°C-67°C and a pressure of 1.33 kPa-1.35 kPa to collect fractions with a yield of 95%; after drying, the fractions were identified as ethyl palm...
Embodiment 3
[0020] Example 3 : In a 250 ml round bottom flask, add 25.64 g (100 mmol) palmitic acid, 14.58 ml (250 mmol) absolute ethanol, 6.52 ml (60 mmol) cyclohexane, 12.82 g NKC-9 macroporous dry hydrogen catalytic resin , heated to reflux at 75°C under agitation, the water separator continued to leak water, and stopped the reaction when no more water was separated; the reaction solution was filtered to separate the resin, and the resin was washed with cyclohexane (20 ml for 2 times), separate and recover the resin, the washing liquid and the filtrate are combined to be separated; the reaction liquid is distilled at 80°C~90°C under normal pressure, and excess ethanol and cyclohexane are distilled off (ethanol and cyclohexane can be further recycled and reused), The resulting reaction solution was then distilled under reduced pressure at 73°C~75°C under a pressure of 2.00 kPa~2.05 kPa to collect fractions with a yield of 90%; after drying, the fractions were identified as ethyl palmit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com