Method for synthetizing clarithromycin intermediate
A technology of clarithromycin and its synthesis method, which is applied in the field of synthesis of clarithromycin intermediates, can solve the problems of pyridine corrosive equipment, safety and environmental hazards, pyridine explosion, etc., and achieve the elimination of major safety hazards, safe and reliable production, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
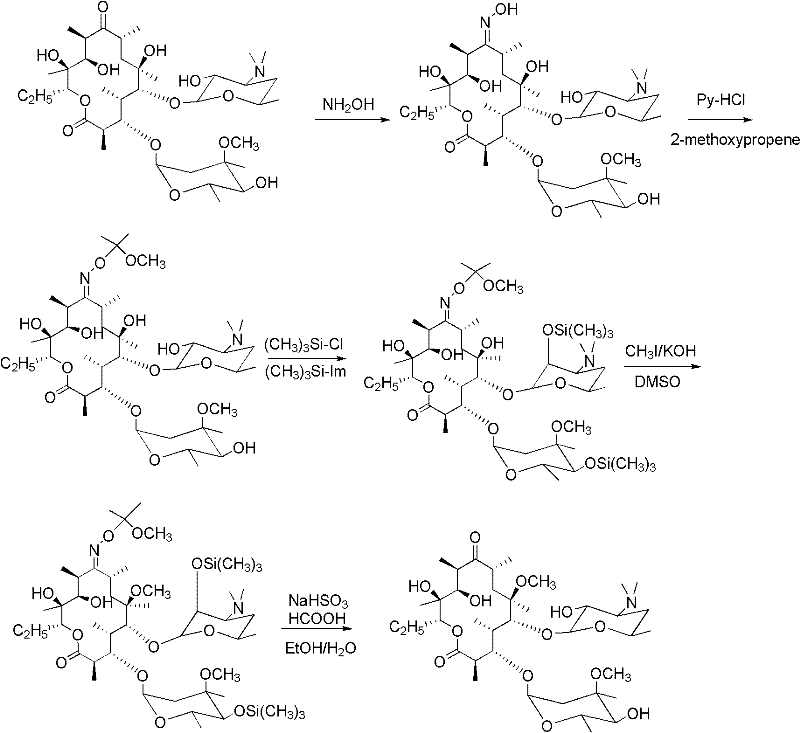
[0035] According to the amount of substance, lactam: concentrated hydrochloric acid is 1.0: 1.0 feeding, wherein N-methyl-pyrrolidone (9.9g, 0.1mol), concentrated hydrochloric acid (10g, 0.1mol), organic solvent absolute ethanol is 40mL, stir at room temperature After 4 hours, the solvent was removed by rotary evaporation, and then 20 mL of absolute ethanol was added, and then rotary evaporation was repeated, the above operation was repeated 3 times, and the filter cake was filtered with suction. The resulting filter cake was a white solid, which was dried to obtain 12.5 g of N-methyl-pyrrolidone hydrochloride The yield was 92%.
[0036] According to the amount of substance, lactam: concentrated hydrochloric acid is 1.0: 1.0 feeding, wherein N-ethyl-pyrrolidone (11.3g, 0.1mol), concentrated hydrochloric acid (10g, 0.1mol), organic solvent absolute ethanol is 40mL, stir at room temperature After 6 hours, the solvent was removed by rotary evaporation, then 20 mL of absolute etha...
Embodiment 2
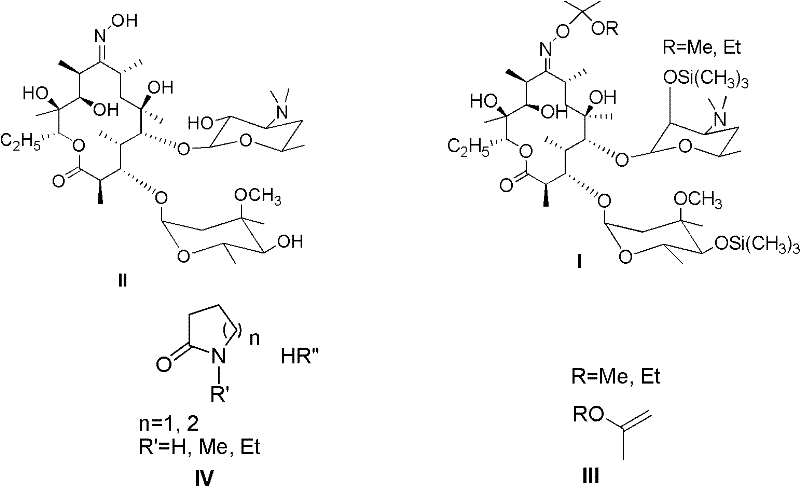
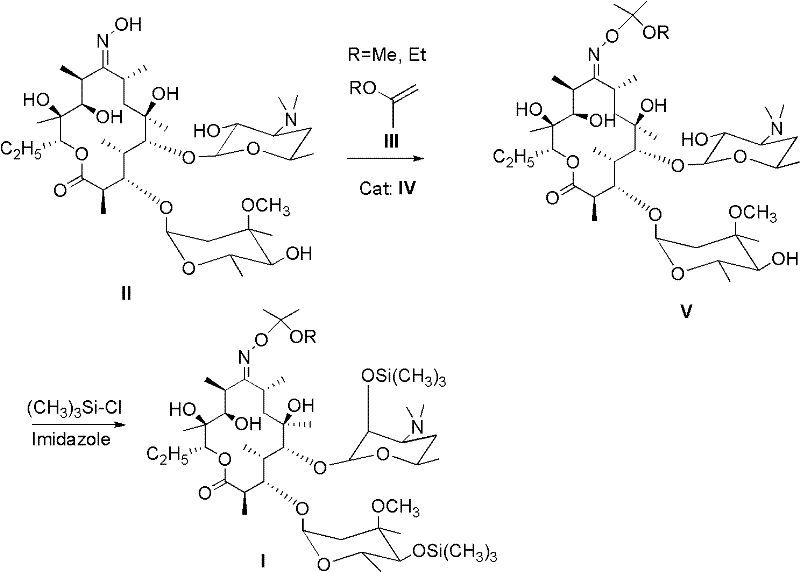
[0043] According to the amount of substance ratio erythromycin A-9-oxime, lactam salt and etherifying agent are 1.0: 1.5: 3.0 feed intake, wherein erythromycin A-9-oxime is 10g (0.0137mol, purity is 94.5%), 2.78 g (0.0205 mol) of N-methyl-pyrrolidone hydrochloride, 5.3 g (0.041 mol) of 2-ethoxypropene, 120 mL of organic solvent dichloromethane, 3.15 g of imidazole, and 4 mL of trimethylchlorosilane.
[0044] First, add erythromycin A-9-oxime and 120 mL of dichloromethane into the reaction flask, turn on the mechanical stirring, heat to 40 ° C, cool to room temperature, add N-methyl-pyrrolidone hydrochloride, and then drop 2 -Ethoxypropene, after the dropwise addition, control the temperature between 20-25 degrees Celsius, after 2 hours of reaction, add imidazole and trimethylchlorosilane, control the temperature at 25-30 degrees Celsius, after 1 hour of reaction, add 40mL of water to stop reaction. The system is layered, take the dichloromethane layer, add 50mL saturated brin...
Embodiment 3
[0046] Ratio erythromycin A-9-oxime by substance amount, lactam salt and etherifying agent are 1.0: 2.0: 3.0 feeding intake, wherein erythromycin A-9-oxime is 10g (0.0137mol, purity is 94.5%), 3.71g (0.0274mol) of N-methyl-pyrrolidone hydrochloride, 4.76g (0.041mol) of 2-methoxypropene, 80mL of organic solvent N, N-dimethylformamide, 3.15g of imidazole, trimethyl Chlorosilane was 4 mL.
[0047] Add N-methyl-pyrrolidone hydrochloride to the solution of erythromycin A-9-oxime in N,N-dimethylformamide, then add 2-methoxypropene dropwise, after the dropwise addition, control the temperature Between 10 and 15 degrees Celsius, after reacting for 3 hours, add imidazole and trimethylchlorosilane, control the temperature between 20 and 25 degrees Celsius, and after reacting for 2 hours, add 30 mL of water to stop the reaction. The reaction solution was not separated after standing, adding 30mL of ethyl acetate to extract, repeating the operation 3 times, combining the ethyl acetate la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com