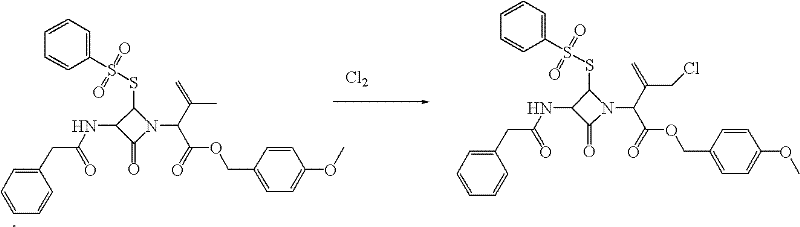
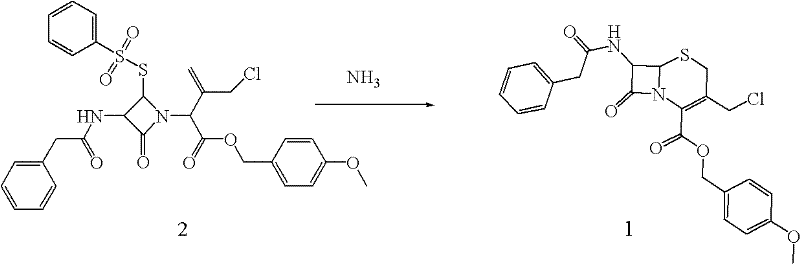
Preparation method of cephalosporin nucleus intermediate
An intermediate and mother nucleus technology, applied in the field of preparation of cephalosporin nucleus intermediates, can solve the problems of great influence on the purity of downstream cephalosporin nucleus, destruction of cephalosporin nucleus, difficulty in product purification, etc., and achieves high GCLE content and reduced The effect of decomposing and reducing the generation of chlorinated by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of Compound 2:
[0050] Put 32g of compound 3 (0.0539moL) into a 2000mL three-necked flask, stir and dissolve with 1000mL of dioxane, add catalyst 1,2-propylene oxide 10.6mL (0.15moL) and absolute ethanol 9.8mL (0.15moL), both The mixing ratio is 1:1, the temperature is lowered to -5°C, chlorine gas is slowly introduced, and when the residual raw material compound 3 is determined to be ≤0.5wt% (HPLC detection), the chlorination reaction is completed, and the solvent is recovered under reduced pressure to obtain a light yellow liquid (compound 2) 36.8g (with a small amount of solvent residue). (HPLC purity analysis: wherein monochloride 88.5wt%, dichloride 1.45wt%, polychloride 0.9wt%).
[0051] Preparation of Compound 1:
[0052] Put the prepared compound 2 into a 500mL three-necked flask, add 120mL of N,N-dimethylformamide (DMF) and stir to dissolve, add CaCl 2 (H 2 O) 2 5.33g (0.036moL), ammonium chloride 1.78g (0.033moL). The temperature was lowere...
Embodiment 2
[0054] Preparation of Compound 2:
[0055] Put 0.15moL of compound 3 into a 3000mL three-necked flask, stir and dissolve with 2000mL ethyl acetate, add 20.6mL (0.30moL) of epichlorohydrin catalyst and 0.15moL of anhydrous isopropanol, the mixing ratio of the two is 2:1, and cool down to 0 ℃, slowly feed chlorine gas, and measure the residual raw material compound 3≤0.5wt% (HPLC detection), the chlorination reaction ends, and the solvent is recovered under reduced pressure to obtain 105.2 g of light yellow liquid (compound 2) (containing a small amount of solvent residue). (HPLC purity analysis: wherein monochloride 89.1wt%, dichloride 1.42wt%, polychloride 0.86wt%).
[0056] Preparation of Compound 1:
[0057] Put the prepared compound 2 into a 3000mL three-necked flask, add 2500mL tetrahydrofuran (DMF) and stir to dissolve, add 2.6g (0.4moL) of CaO and 1.78g (0.8moL) of ammonium carbonate. The temperature was lowered to -15°C, and 0.18 mol of ethylamine was added dropwise. ...
Embodiment 3
[0059] Preparation of Compound 2:
[0060] Put 96g (0.16moL) of compound 3 into a 5000mL three-necked flask, stir and dissolve it with 3000mL of diethylene glycol monoethyl ether, add 10.6mL (0.9moL) of catalyst ethylene oxide and 6.0moL of acetic acid, cool down to 15°C, and slowly introduce chlorine gas, When it is determined that the residual raw material compound 3≤0.5wt% (HPLC detection), the chlorination reaction is completed, and the solvent is recovered under reduced pressure to obtain 120 g of a light yellow liquid (compound 2) (containing a small amount of solvent residue). (HPLC purity analysis: wherein monochloride 91wt%, dichloride 1.40wt%, polychloride 0.86wt%).
[0061] Preparation of Compound 1:
[0062] Put the prepared compound 2 into a 3000mL three-neck flask, add 2500mL of 1,3-dimethyl-2-imidazolonetetrafluoroboron and stir to dissolve, add 1.6moL of calcium hydroxide and 0.2moL of ammonium acetate. The temperature was lowered to -5°C, and 2.5 moL of ammo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com