Lomefloxacin hydrochloride eye drops and preparation method and application thereof
A technology of lomefloxacin hydrochloride and eye drops, applied in the field of medicine, can solve the problems of low medication compliance of patients, bitter taste of medicine, loss of medicine liquid, etc., to improve bioavailability, prolong residence time, and ensure full absorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
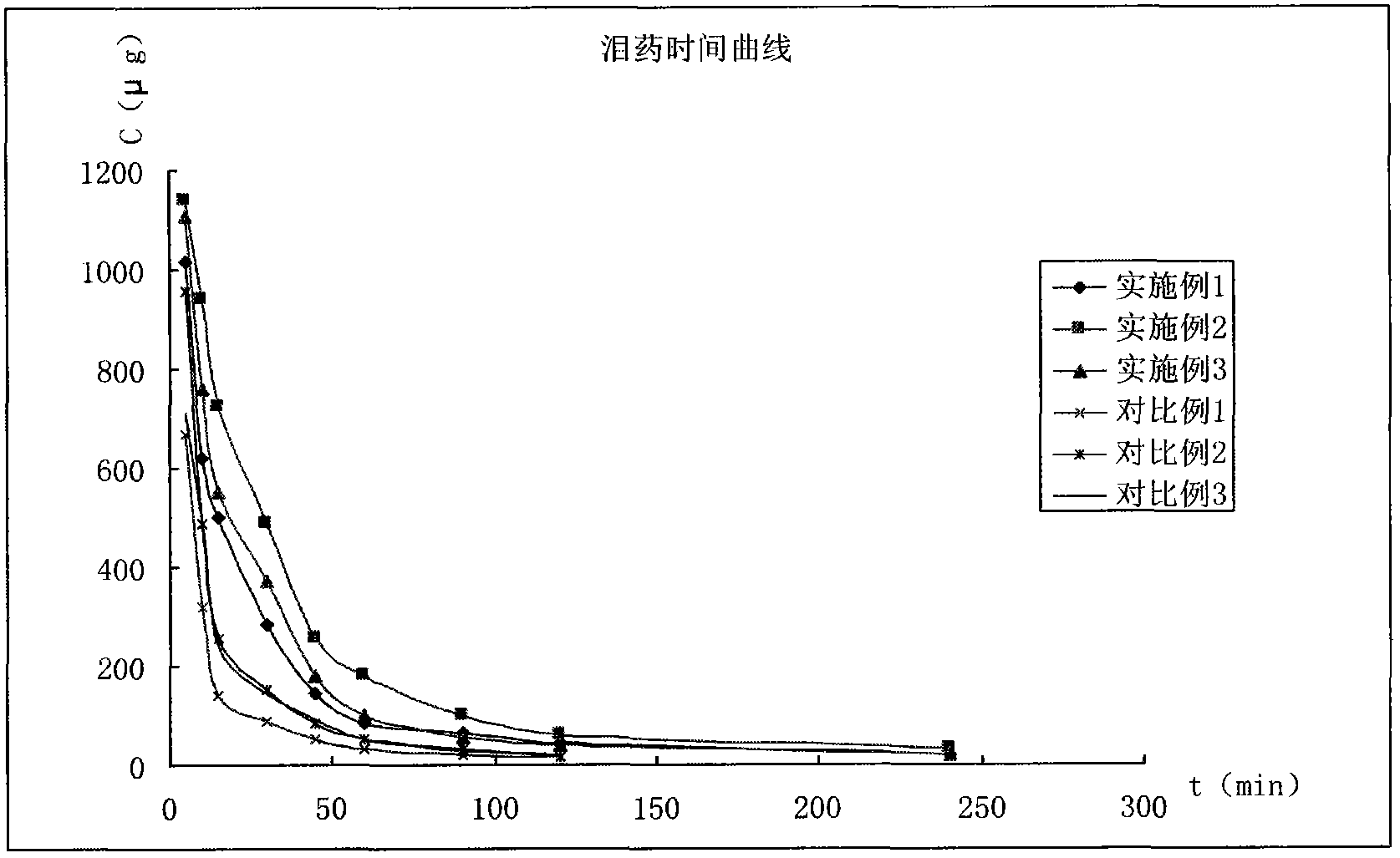
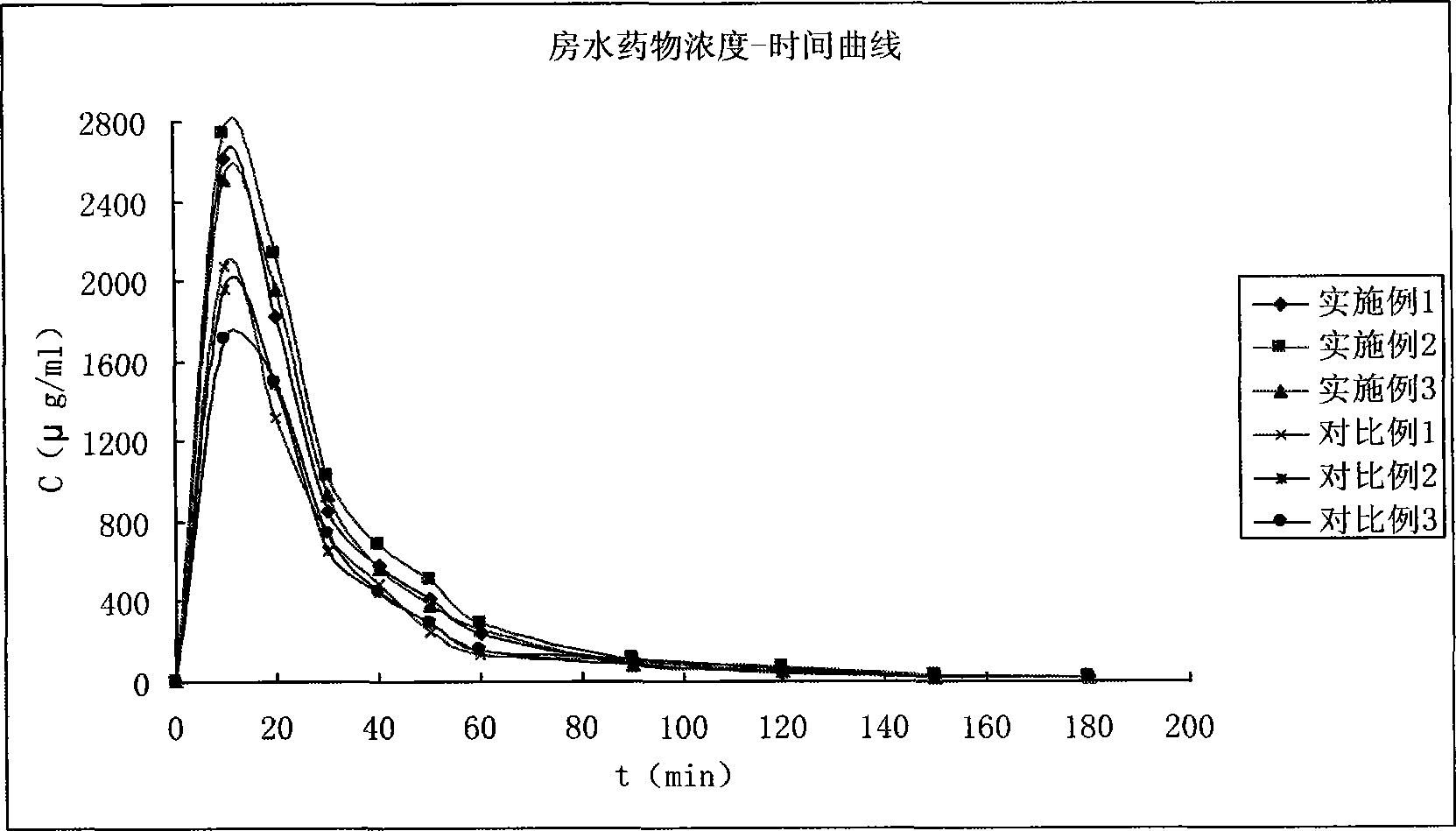
Image
Examples
Embodiment 1
[0026] prescription:
[0027]
[0028] Its preparation method is:
[0029] (1) Disperse the prescribed amount of sodium hyaluronate in an appropriate amount of water for injection at 40°C to 55°C to swell to make a transparent solution;
[0030] (2) Dissolve the prescribed amount of main drug lomefloxacin hydrochloride in an appropriate amount of water for injection at 60°C to 70°C, add the prescribed amount of sodium dihydrogen phosphate-disodium hydrogen phosphate buffer, sodium chloride, benzalkonium bromide, The mixed solution of glycerin is stirred and mixed, and then transferred to (1), the pH is adjusted to 6.0 with 0.1mol / L sodium hydroxide solution, the full amount of water for injection is added, and filtered with a 0.22μm microporous membrane. After passing the inspection, no Bacteria packaged.
Embodiment 2
[0032] prescription:
[0033]
[0034] Its preparation method is:
[0035] (1) Disperse the prescribed amount of sodium hyaluronate in an appropriate amount of water for injection at 40°C to 55°C to swell to make a transparent solution;
[0036] (2) Dissolve the prescribed amount of the main drug lomefloxacin hydrochloride in an appropriate amount of water for injection at 60°C to 70°C, add the prescribed amount of sodium dihydrogen phosphate-disodium hydrogen phosphate, sodium chloride, benzalkonium bromide, and glycerin Mix the solution, stir and mix, then transfer to (1), adjust the pH to 6.0 with 0.1mol / L sodium hydroxide solution, add the full amount of water for injection, filter with a 0.22μm microporous membrane, and pack aseptically after passing the inspection Instantly.
Embodiment 3
[0038] prescription:
[0039]
[0040] Its preparation method is:
[0041] (1) Disperse the prescribed amount of sodium hyaluronate in an appropriate amount of water for injection at 40°C to 55°C to swell to make a transparent solution;
[0042] (2) Dissolve the prescribed amount of the main drug lomefloxacin hydrochloride in an appropriate amount of water for injection at 60°C to 70°C, add the prescribed amount of sodium dihydrogen phosphate-disodium hydrogen phosphate, sodium chloride, benzalkonium chloride, and glycerin Mix the solution, stir and mix, then transfer to (1), adjust the pH to 6.0 with 0.1mol / L sodium hydroxide solution, add the full amount of water for injection, filter with a 0.22μm microporous membrane, and pack aseptically after passing the inspection Instantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com