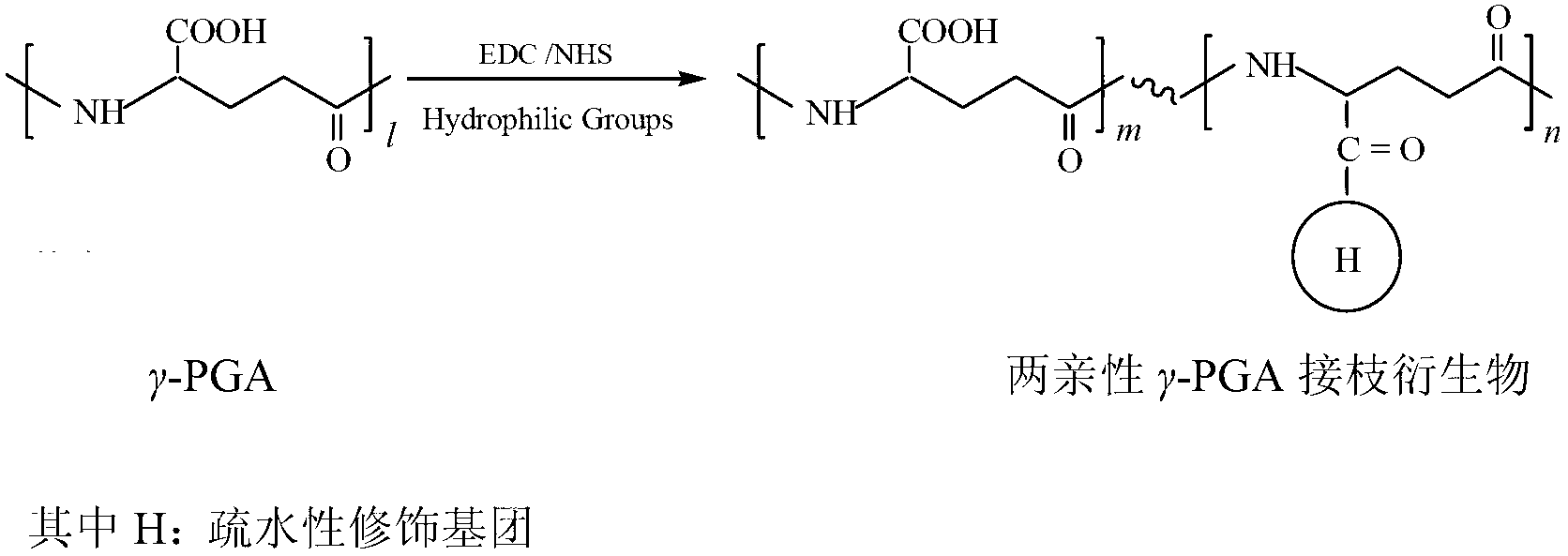
Preparation method of amphipathic gama-polyglutanmic acid nanodrug carrier
A nano-drug carrier, polyglutamic acid technology, applied in the field of polymers and medical biomaterials, can solve problems such as limitations, weakened ability to encapsulate drugs, and poor self-assembly performance of modified products, and achieve easy operation and simple process , the effect of controllable output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A method for preparing amphiphilic gamma-PGA nano drug carrier, the method comprises the following steps.
[0049] (1) Dissolve 1g γ-PGA (molecular weight 380KDa, molecular weight distribution coefficient 1.3) in 100mL DMSO (solvent I) at room temperature to form a transparent 10g / L γ-PGA solution.
[0050] (2) Add EDC to the above-mentioned γ-PGA solution in an amount whose ratio to the amount of glutamic acid monomer residues in the γ-PGA obtained in step (1) is 1.2, and heat at room temperature at 500r / min The reaction was activated for 12 hours to form a reaction activation solution.
[0051] (3) Weigh cholesterol according to the ratio of 0.4 to the amount of glutamic acid monomer residues in γ-PGA obtained in step (1), dissolve it in 100 mL of DMSO-pyridine mixed in equal proportions (solvent II) middle.
[0052] (4) Slowly add the cholesterol-containing solution obtained in step (3) dropwise to the reaction activation solution obtained in step (2) within 30 min...
Embodiment 2
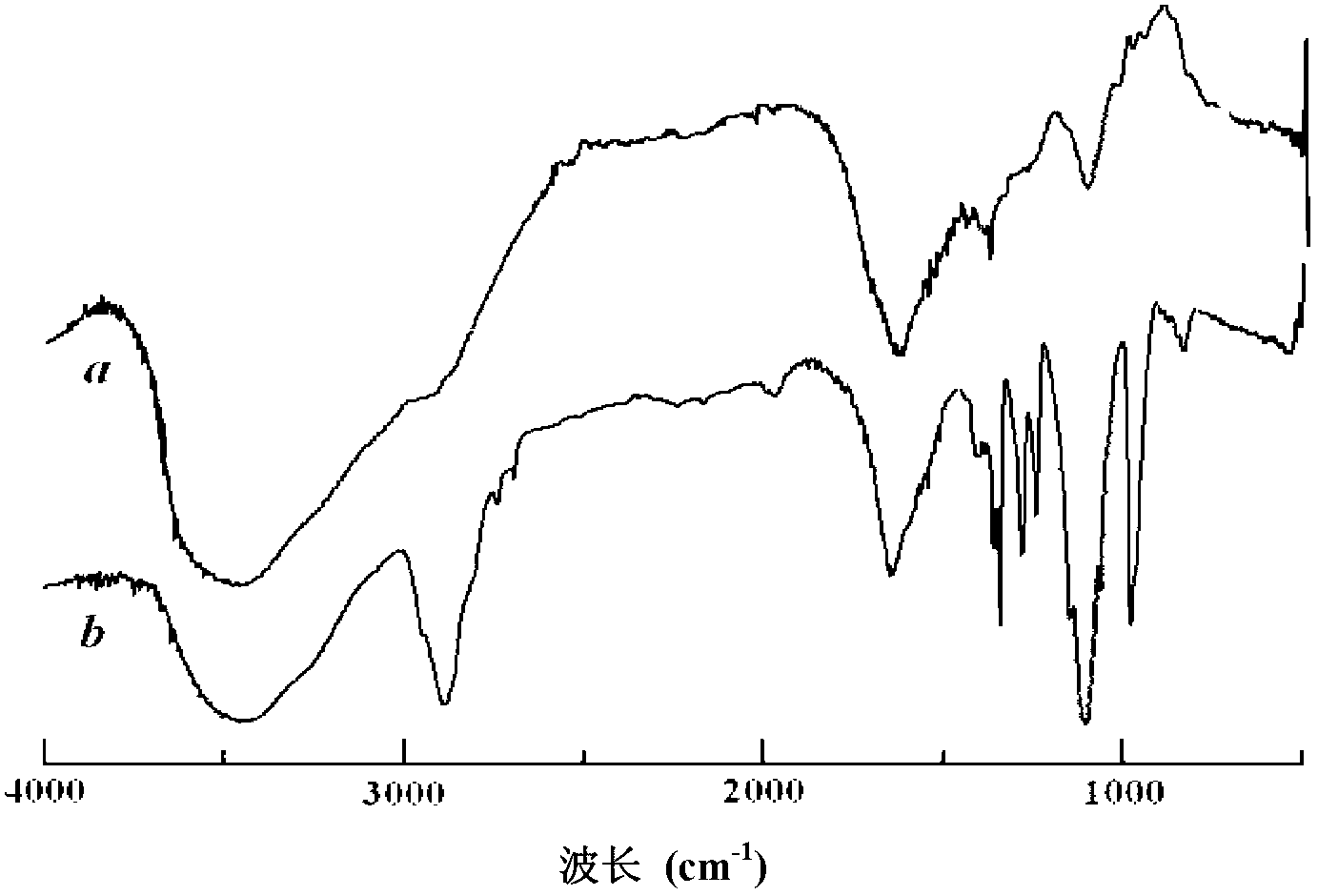
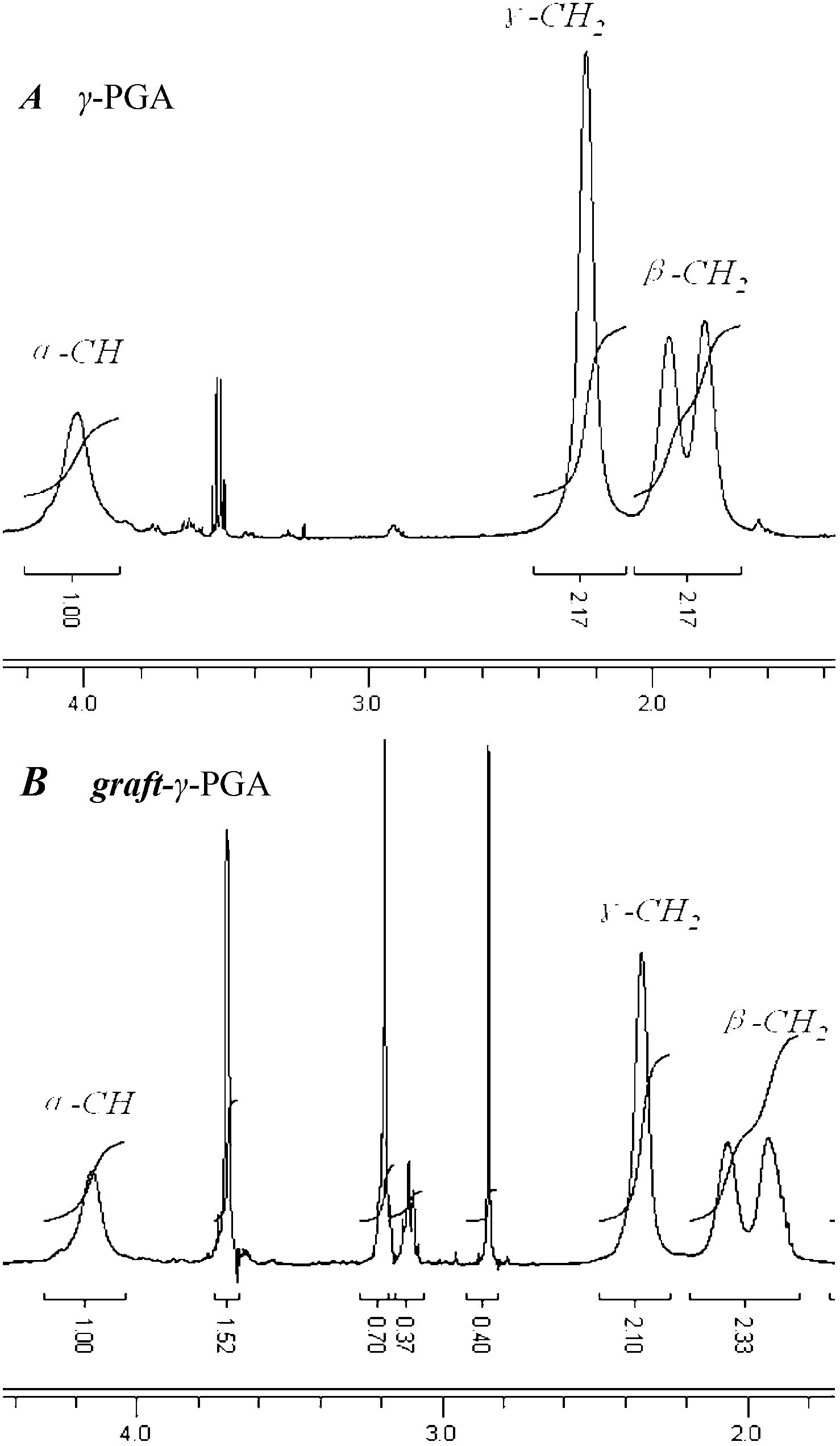
[0055] Solvent I uses 1.0mol / L phosphate buffer saline (PBS), the ratio of the amount of catalyst EDC to the amount of glutamic acid monomer residues in the γ-PGA is 2.0, the amount of cholesterol and the amount of glutamic acid in the γ-PGA are Except that the substance amount ratio of the acid monomer residue is 1.0, other conditions are the same as in Example 1. The FTIR and 1 H-NMR such as figure 2 (b) and image 3 (B) shown. according to 1 H-NMR spectrum analysis shows that the degree of substitution of side chains is about 37%.
[0056] The amphiphilicity and critical micelle concentration (CMC) of the γ-PGA graft modified products prepared under this condition were investigated by the pyrene fluorescent probe method, and the pyrene emission spectra were as follows: Figure 4 Shown, the characteristic peak ratio (I 372 / I 385 ) and the logarithmic plot of the concentration of γ-PGA grafted modification Figure 5 , the concentration corresponding to the turning p...
Embodiment 3
[0058] Add the synthetic product of Example 1 to 1 mol / L PBS buffer solution with pH 4.0 so that the concentration of the synthetic product is 1 mg / mL, shake at 25°C for 12 hours at a low speed, and then use a probe-type ultrasonic instrument in an ice-water bath Ultrasonic treatment, working for 2s, stopping for 2s, ultrasonic time for 2min, repeated 3 times to obtain a dispersion of amphiphilic γ-PGA nanomicelles, and then centrifuged at 14000g to collect the precipitate-freeze-drying step to obtain amphiphilic γ-PGA nanomicelle. Electron microscope observation and particle size analyzer results showed that the shape of amphiphilic γ-PGA nanomicelles was relatively regular; the average particle size was about 220nm, and the particle size polydispersity index (PDI) was 0.35.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com