Method for extracting and purifying phloretin from Malus toringoides(Rehd.) Hughes. and Malus tiansitoria(Batal.)Schneid.
A purification method, the technology of phloretin, applied in the field of medicine, to achieve the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 The separation and purification method of phloretin of the present invention
[0022] (1) Take 8 kg of dried leaves of Begonia variegata, crush or crush them by hand, weigh about 1 kg each time, add 5 times of 95% ethanol for reflux extraction, extract twice, filter, collect the subsequent filtrate, and recover under reduced pressure to obtain Ethanol site extract 563g. Add water to dissolve the extract of the ethanol part, then add 2 times of ethyl acetate and extract repeatedly until the ethyl acetate solution is colorless, combine the ethyl acetate solution, recover under reduced pressure to obtain 240 g of the extract of the ethyl acetate part.
[0023] (2) Dissolve the ethyl acetate part with an appropriate amount of methanol, and at the same time add 200g of silica gel (200-300 mesh) to mix the sample. Take 3000g of silica gel for wet packing, add the sample after the silica gel column is uniformly precipitated, and use chloroform-methanol=50: (1-3) ...
Embodiment 2 Embodiment 1
[0026] The structure identification of embodiment 2 embodiment 1 gained product
[0027] The phloretin obtained in Example 1 is carried out for structural identification, and the data are as follows:
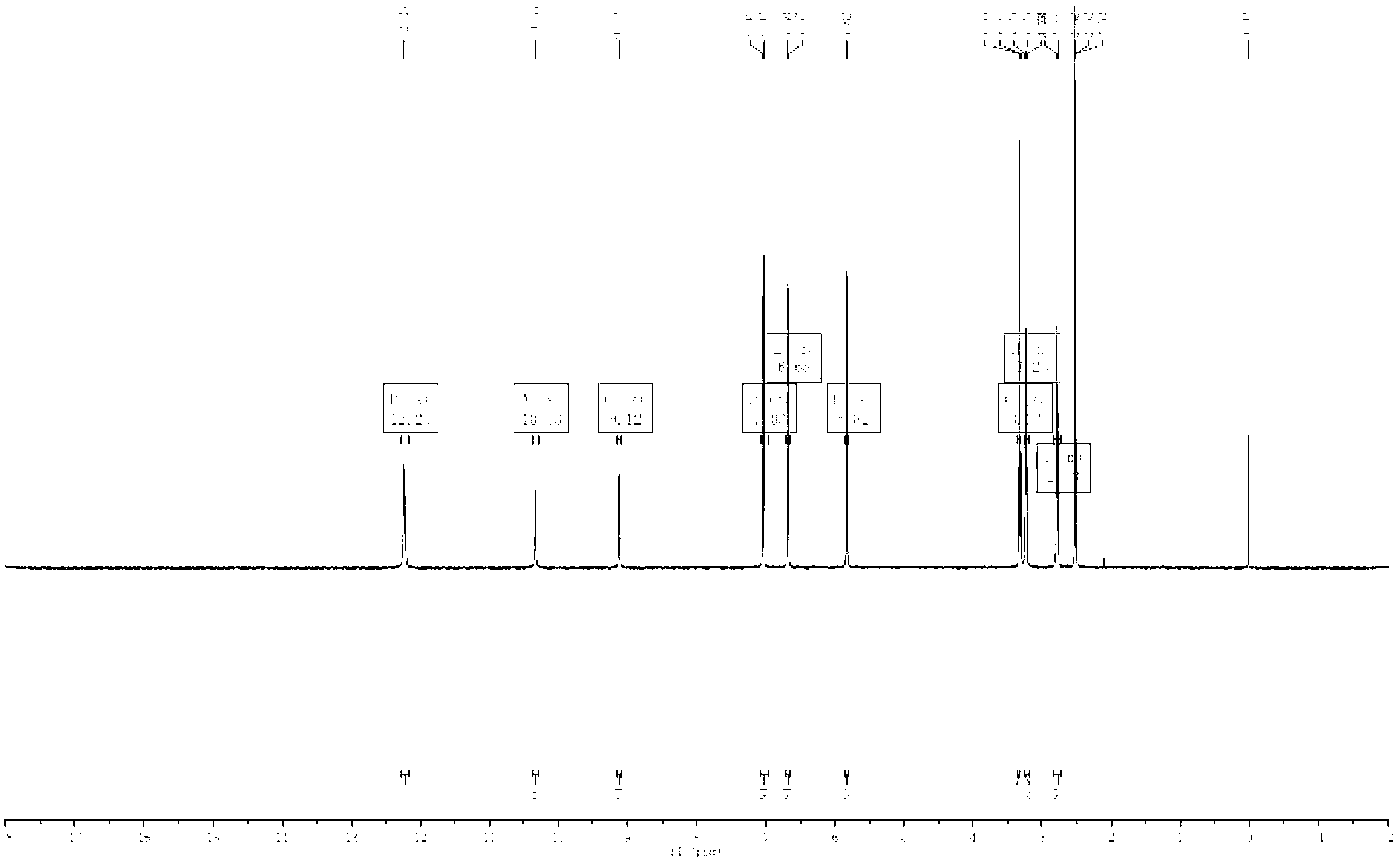
[0028] This product is a light pink powder (methanol), sprayed with aluminum trichloride color developer under TLC ultraviolet lamp to display bright blue. The reaction of magnesium hydrochloride powder was positive, and it was preliminarily judged to be flavonoids or flavonols. Molecular formula: C 15 h 14 o 5
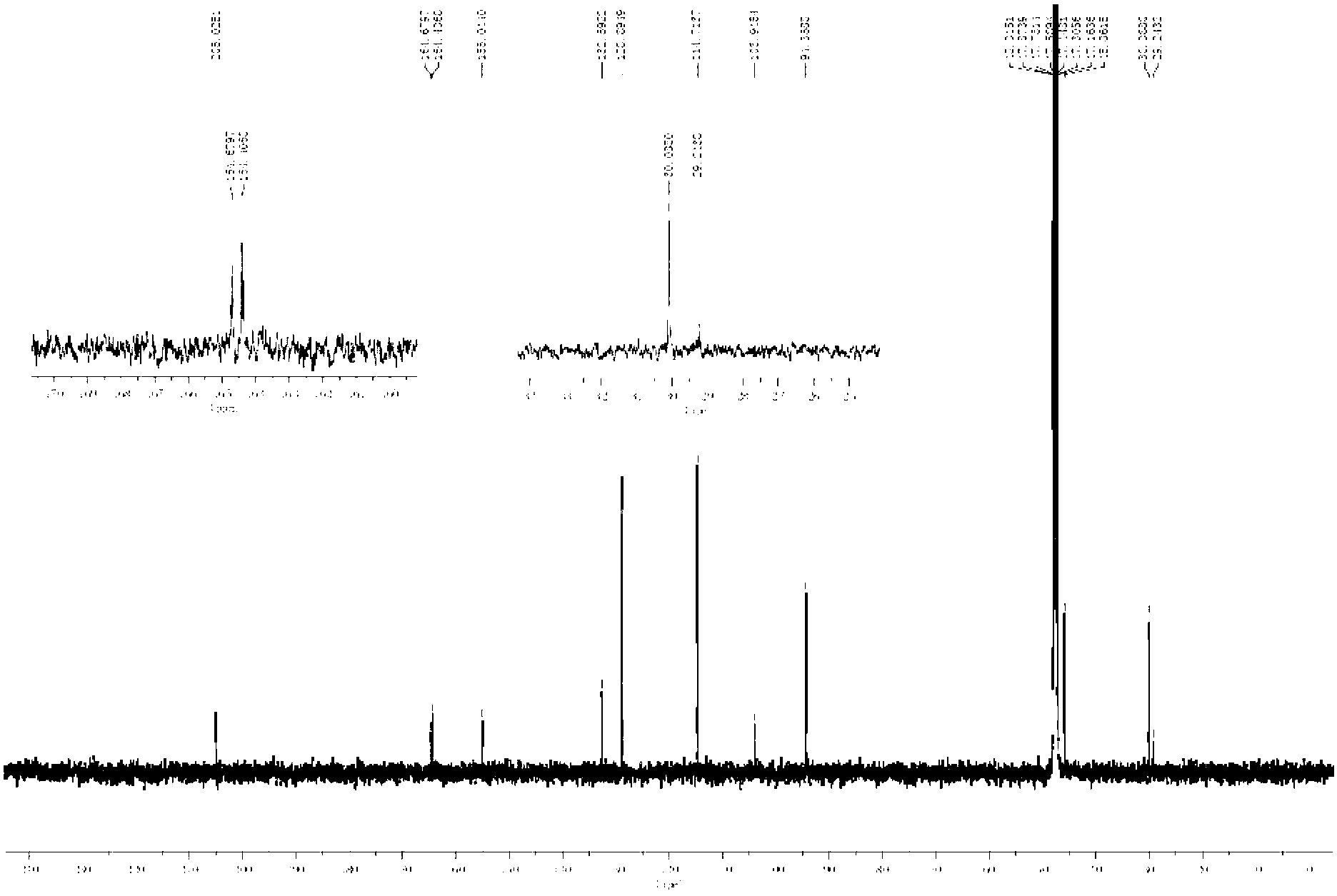
[0029] 1H-NMR (600MHz, DMSO-d612.23 (2H, s, 2', 6'-OH), 10.33 (1H, s, 4'-OH), 9.12 (1H, s, 4-OH), 7.03 ( 2H,s,H-3',5'),6.68(2H,d,J=8.5Hz),5.82(2H,s,H-3',5'),3.23(2H,d,J=7.6Hz ),2.78(2H,d,J=7.6Hz),13C-NMR(151MHz,DMSO-d6)205.0(C=O),164.7(C-4'),164.4(C-2',C-6' ),155.0(C-4),132.5(C-1),128.9(C-2,C-6),114.7(C-3,C-5)103.9(C-1')94.4(C-3' ,C-5'), 45.9(C-α), 30.0(C-β) were identified as the compound Phloretin by comparison with literature data.
[0030]
Embodiment 3
[0031] The purity analysis of the phloretin prepared by the method of the present invention in embodiment 3
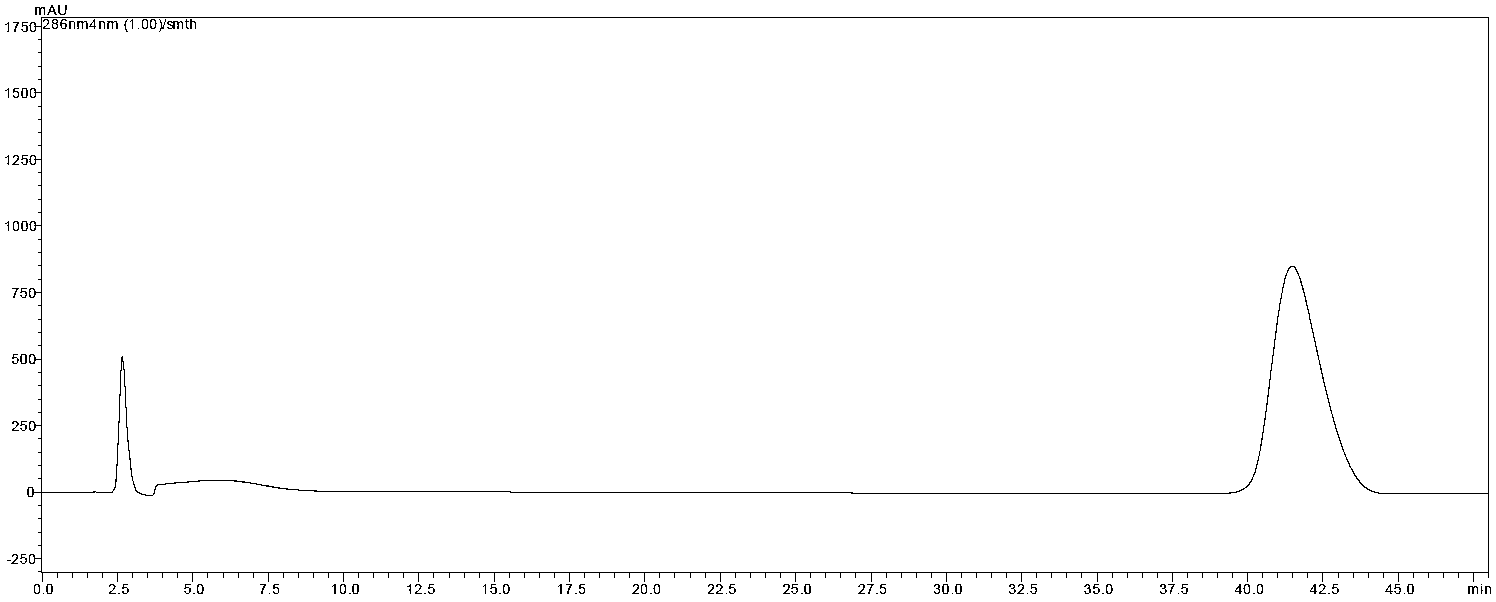
[0032] The purity of the phloretin prepared in Example 1 is detected, and the conditions are as follows:
[0033] Instruments: Agilent1200 high-performance liquid chromatography, DiamonsilTM C18HPLC column (250×4.6mm, 5μm), BP211D electronic analytical balance (1 / 100,000, Sartorius Germany Sartorius AG).
[0034] Mobile phase: 27% acetonitrile-water
[0035] Detection wavelength: 286nm
[0036] Temperature: 30 degrees Celsius
[0037] Injection volume: 20μl
[0038] Elution condition: isocratic elution
[0039] result:
[0040] As determined by high performance liquid chromatography, the purity of the phloretin prepared by the method of the present invention reaches 98.61%, and the recovery rate is 65.12%.
[0041] The present invention does not adopt enzymatic hydrolysis or acid hydrolysis, and for the first time, phloretin is isolated from the plants of the Ros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com