Method for determining ephedrine hydrochloride content in lung-clearing inflammation pill by high-performance liquid phase
A technology for ephedrine hydrochloride and its determination method, which is applied in the field of ephedrine hydrochloride content, and can solve the problems of poor reproducibility of thin-layer scanning method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] prescription:
[0100] 250 parts of ephedra, 750 parts of gypsum, 750 parts of earthworm
[0101] 250 parts of burdock seed, 250 parts of tingli seed, 100 parts of bezoar
[0102] Bitter almond (fried) 60 parts Antelope horn 30 parts
[0103] Preparation:
[0104] The above eight flavors, except the artificial bezoar, the antelope horn is crushed into fine powder, and the other six flavors such as ephedra are crushed into fine powder, blended with the above antelope horn and artificial bezoar powder, mixed, and sieved. For every 100g of powder, use 60~80g of refined honey and add appropriate amount of water pan pills to make water honey pills;
Embodiment 2
[0106] (1) Preparation of test solution:
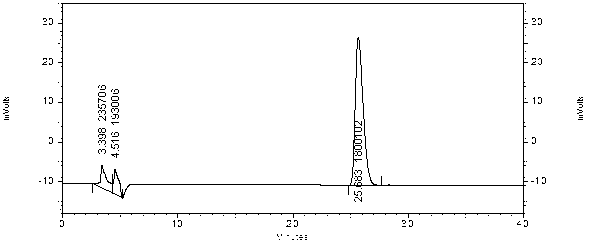
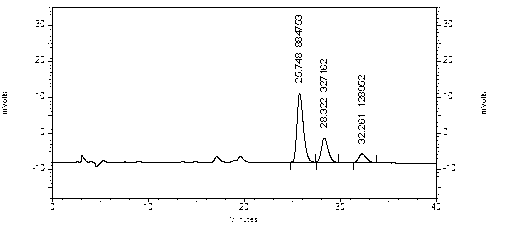
[0107] 1) Preparation of reference substance solution: Take an appropriate amount of ephedrine hydrochloride reference substance, weigh it accurately, add methanol to make a solution containing 30 μg per 1 ml, and obtain it;
[0108] 2) Preparation of the test solution: take Qingfei Xiaoyan Pills, grind them finely, take about 2g, weigh them accurately, add 120ml of 5mol / L sodium hydroxide solution, 7.5g of sodium chloride, ultrasonicate for 10 minutes, and keep slightly boiling Under distillation, use a 5ml measuring bottle filled with 0.5mol / L hydrochloric acid solution to collect 90-98ml of distillate, add water to the mark, shake well, and you get it;
[0109] 3) Preparation of negative control solution: prepare a sample without ephedra according to the prescription and process, and prepare according to the preparation method of the test sample;
[0110] (2) Chromatographic conditions
[0111] Mobile phase: acetonitrile-0.02mol / ...
Embodiment 3
[0117] (1) Preparation of test solution:
[0118] 1) Preparation of reference substance solution: Take an appropriate amount of ephedrine hydrochloride reference substance, weigh it accurately, add methanol to make a solution containing 30 μg per 1 ml, and obtain it;
[0119] 2) Preparation of the test solution: take Qingfei Xiaoyan Pills, grind them finely, take about 2g, weigh them accurately, add 80ml of 5mol / L sodium hydroxide solution and 7.5g of sodium chloride, ultrasonicate for 10 minutes, and keep slightly boiling Under distillation, use a 5ml measuring bottle filled with 0.5mol / L hydrochloric acid solution to collect 90-98ml of the distillate, add water to the mark, shake well, and filter through a 0.45μm microporous membrane to obtain the product;
[0120] 3) Preparation of negative control solution: prepare a sample without ephedra according to the prescription and process, and prepare according to the preparation method of the test sample;
[0121] (2) Chromatogr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com