Alpha-amylase truncated body and application thereof
An amylase and variant technology, which is applied in the field of truncation of α-amylase, can solve problems such as increasing cost, and achieves a technology that is beneficial to industrial production, improves thermal stability and specific activity, large catalytic efficiency and thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
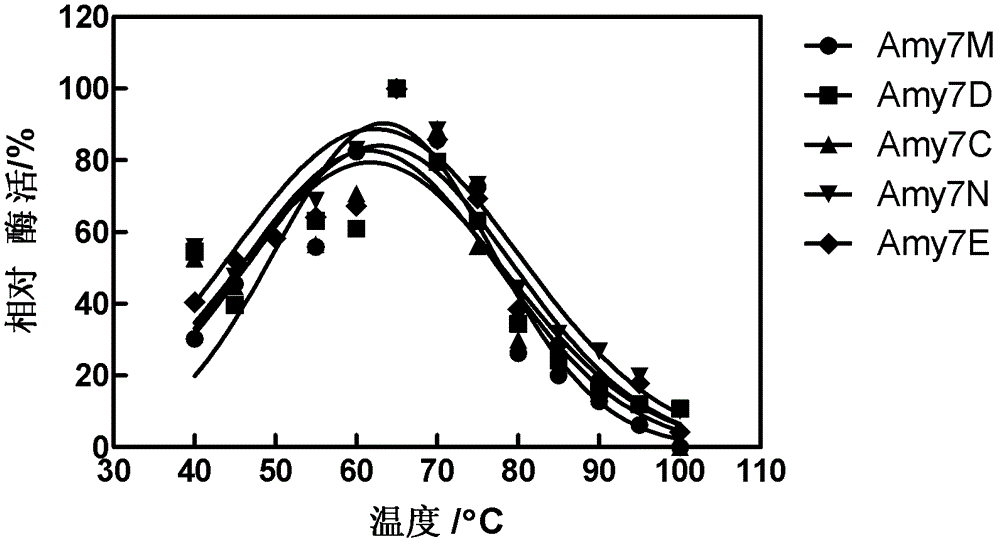
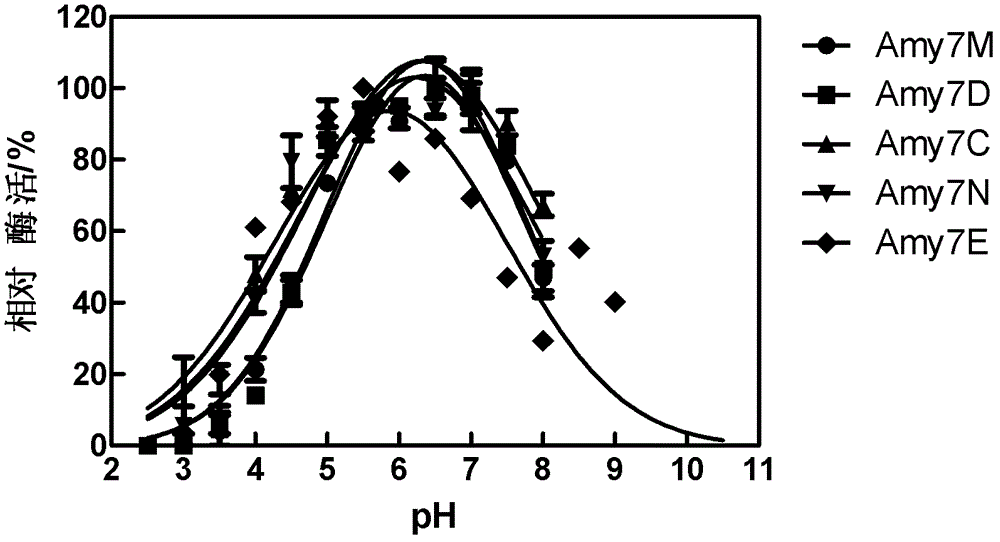
[0043] This example illustrates the purification of the parent enzyme, the characterization of the purified enzyme and the analysis method of the enzymatic hydrolyzate
[0044] 1. Cultivation of Bacillus subtilis CN7
[0045]Bacillus subtilis CN7 was isolated from the soil sample of Guangxi University farm, and the strain was preserved in our laboratory, and submitted to the China Center for Type Culture Collection with the preservation number CCTCC M 2012061. The medium is liquid LB plus 1% soluble starch, and each liter of medium contains 10 g of peptone, 5 g of yeast extract, 10 g of sodium chloride, and 10 g of soluble starch. The solid medium was the above liquid medium with 1.5% agar added. Take a tube of the original strains stored in the cryopreservation tube at -80°C, use an inoculation loop to get the bacteria to streak on the solid medium plate, cultivate overnight in a 37°C incubator, pick a single colony and inoculate it in the liquid medium , placed on a consta...
Embodiment 2
[0068] This example illustrates the cloning and analysis of the full-length α-amylase gene, the construction and expression of the recombinant plasmid, and the purification of the recombinant enzyme
[0069] 1. Cloning of full-length α-amylase gene and construction of recombinant plasmid
[0070] With reference to "Molecular Cloning Experiment Guide" (Sam Brook, Russell. Molecular Cloning Experiment Guide [M]. Beijing: Science Press, 2002.), extract the total DNA of Bacillus subtilis CN7, use this total DNA as a template, and use primers Amy7-S and Amy7-A, the complete α-amylase gene amy7 with a full length of 1980bp was amplified by PCR, and the primer sequences were as follows:
[0071] Amy7-S: 5′-GTA TCATGA TGTTTGAAAAACGATTCAAAAC-3′
[0072] Amy7-A: 5′-GCG AAGCTT AATCAATGCGGAAGATAACCATTC-3′
[0073] For convenience of operation, a PagI restriction site (underlined part) was introduced into the upstream primer Amy7-S, and a Hind III restriction site (underlined part) w...
Embodiment 3
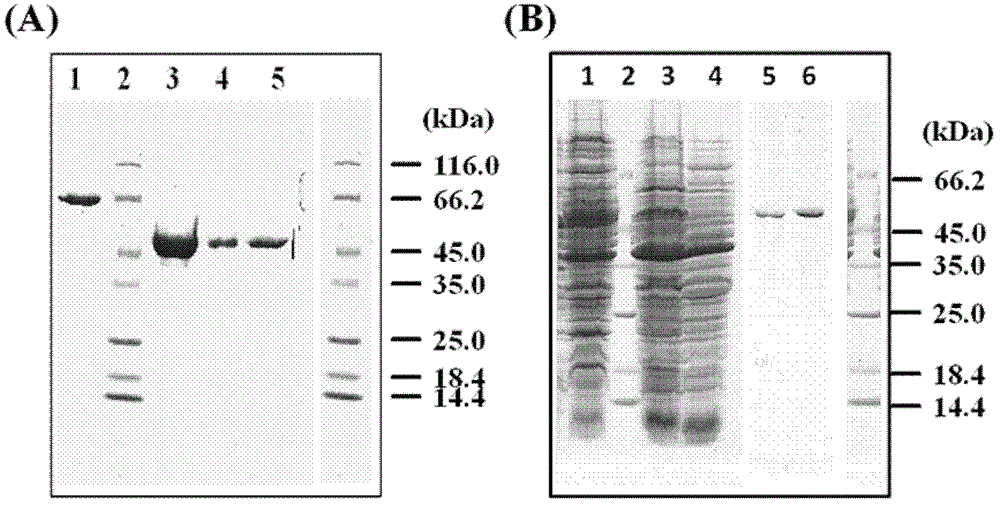
[0082] This example illustrates the construction and purification of truncated α-amylase
[0083] 1. Construction of truncated α-amylase
[0084] Using Amy7M-S and Amy7M-A as primers, using the total DNA of Bacillus subtilis CN7 as a template, adopt the same method as in Example 1, 1, the cloning of the full-length α-amylase gene and the construction of the recombinant plasmid, to construct a gene containing the truncated Amy7M code. Gene recombinant plasmid pSA7M, the primer sequence is as follows:
[0085] Amy7M-S 5`-GAC TCATGA GCTCGGTCAAAAACGGGACCATC-3`
[0086] Amy7M-A 5`-GTAC AAGCTT ATGCGGAAGATAACCATTCAAA-3`
[0087] A Pag I restriction site (underlined part) was introduced into the upstream primer Amy7M-S, and a Hind III restriction site (underlined part) was introduced into the downstream primer Amy7M-A.
[0088] Using primers Amy7D-S and Amy7D-A as primers, and using the total DNA of Bacillus subtilis CN7 as a template, the same method as in Example 1, 1, the cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com