PtBA-b-PEG-b-PtBA block copolymer, and preparation method and application thereof
An a-b-b-b-a, block copolymer technology, applied in the direction of pharmaceutical formulations, drug combinations, medical preparations with non-active ingredients, etc., can solve the problems of easy shedding of target molecules and low polymer targeting efficiency, etc., and achieve excellent hydrophilicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
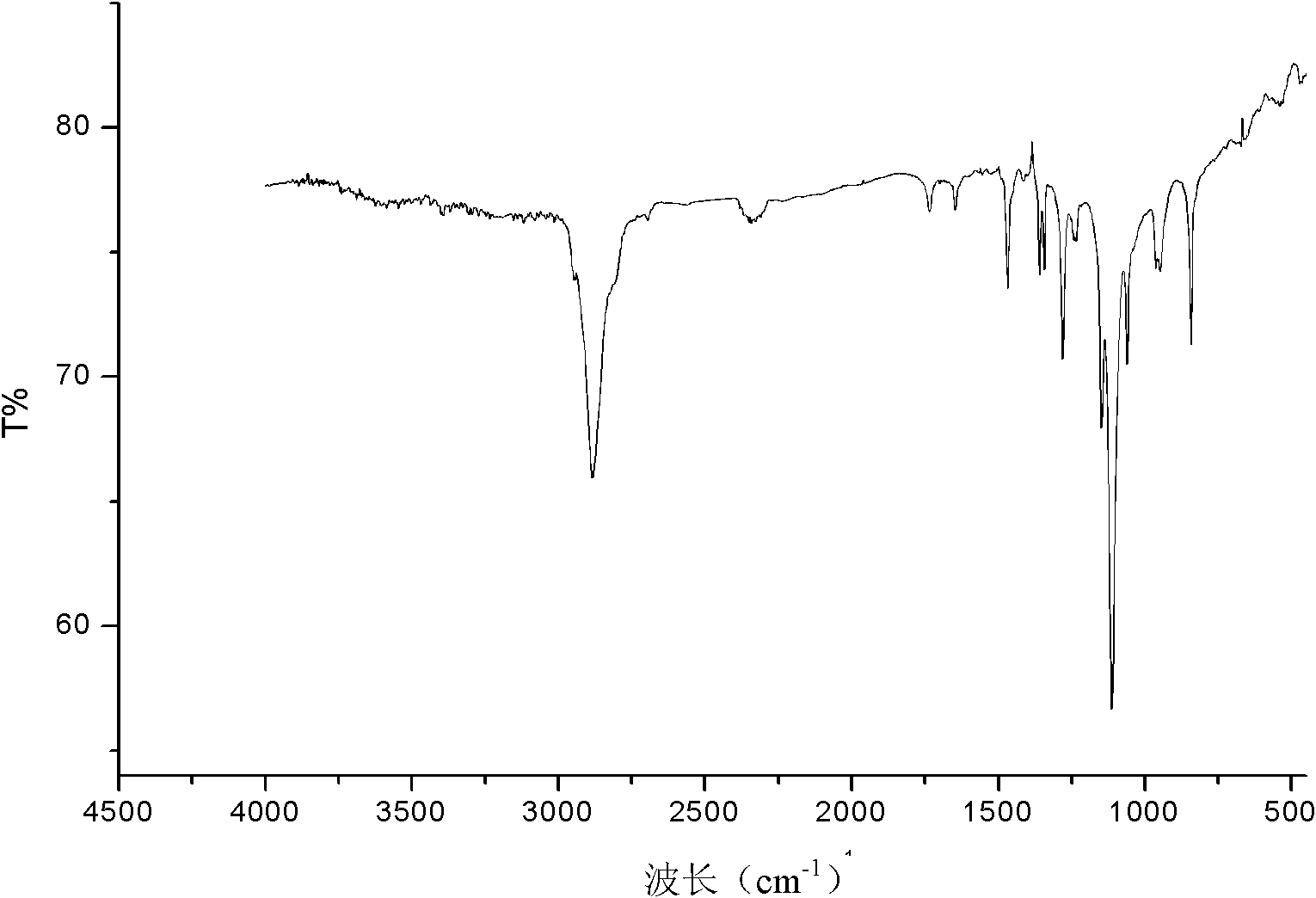
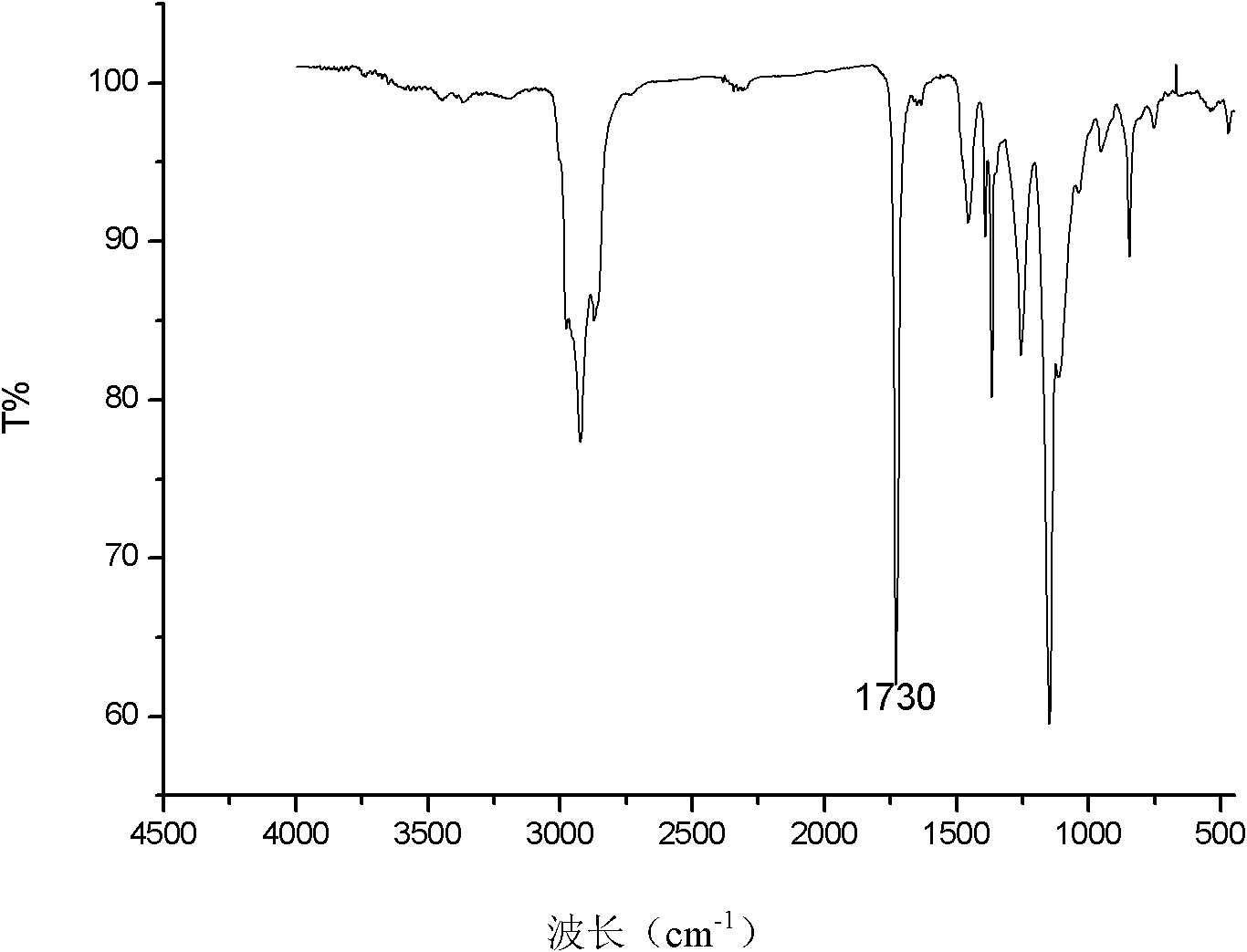
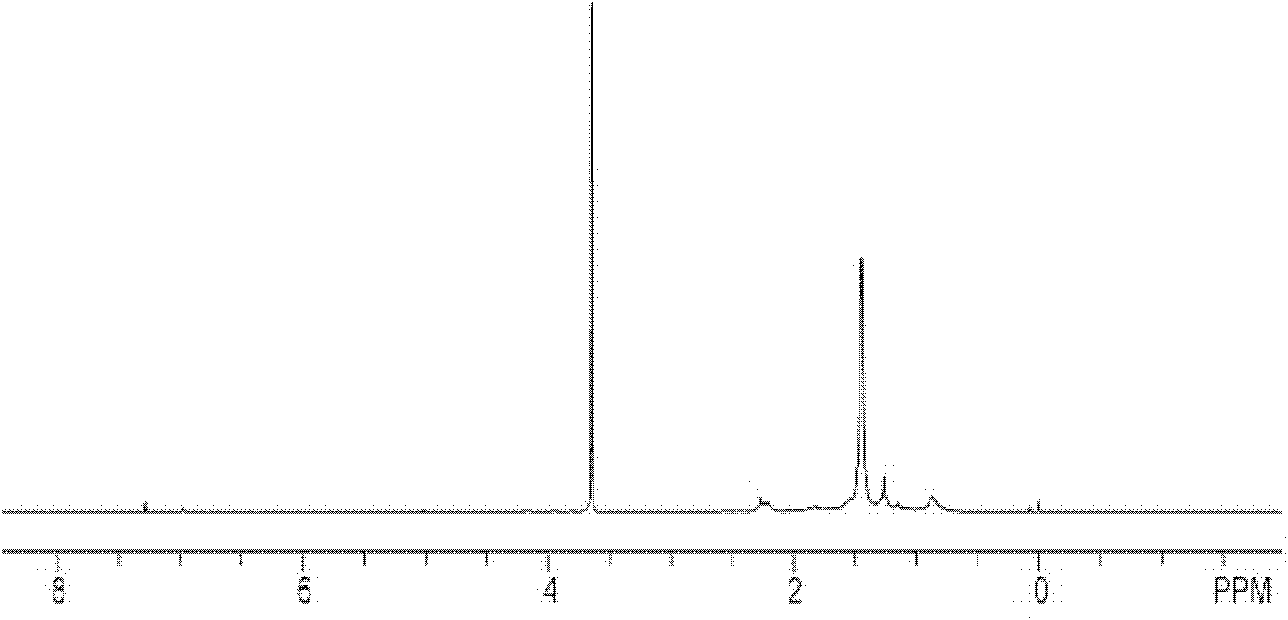
[0037] (1) Preparation of bromopolyethylene glycol macroinitiator: in one or two round-bottomed flasks, under the condition of nitrogen gas, 2 g of polyethylene glycol with a molecular weight of 6000 was dissolved in 20 mL of anhydrous toluene (sodium / diphenyl Ketone system refluxed to remove water), a small amount of water in polyethylene glycol 6000 was removed by azeotropic distillation with toluene. Cool the reaction system to 0°C, add 1.4mL redistilled triethylamine into the flask under stirring, keep the system at 0°C, add 0.124mL 2-bromoisobutyryl bromide dropwise to the flask, and cover the flask with tinfoil Avoid light. After stirring at room temperature for 24 h, the reaction was stopped. The white solid was filtered off, the solvent toluene was removed by rotary evaporation, the crude product was added to water and extracted 3 times with dichloromethane, precipitated with excess anhydrous ether, and dried in vacuo to obtain polyethylene glycol bifunctional bromina...
Embodiment 2
[0044] (1) In the preparation of bromopolyethylene glycol macroinitiator, select dichloromethane as solvent, and the reaction time is 48h, and other is with embodiment 1.
[0045] (2) The generation of block copolymer: device and operation are the same as embodiment 1, with polyethylene glycol macroinitiator 0.2g i.e. 0.03mmol, and monomer tert-butyl acrylate 0.79mL i.e. 5.4mmol, ligand PMDETA13 μ L i.e. Add 0.06mmol into the Schlenk tube, under the protection of nitrogen, freeze in liquid nitrogen-vacuumize-dissolve three times to remove the oxygen in the system; then add the catalyst cuprous bromide 9mg (0.06mmol) Stir in an oil bath at ℃ for 8 hours. After the reaction, the product is diluted with toluene and passed through a neutral alumina column to remove copper salts. Unpolymerized small molecule monomers and solvents are removed under reduced pressure, and vacuum-dried to obtain poly(tert-butylacrylate-polyacrylate) Ethylene glycol-tert-butyl polyacrylate triblock copo...
Embodiment 3
[0047] (1) In the preparation of bromopolyethylene glycol macroinitiator, reacted 36 hours, other are with embodiment 1.
[0048] (2) Generation of block copolymer: device and operation are the same as embodiment 1, with polyethylene glycol macroinitiator 0.2g 0.03mmol, and monomer tert-butyl acrylate 1.23mL i.e. 8.4mmol, ligand PMDETA13 μ L i.e. 0.06 Add mmol to the Schlenk tube, under the protection of nitrogen, freeze in liquid nitrogen-vacuumize-dissolve three times to remove the oxygen in the system; then add the catalyst cuprous bromide 9mg (0.06mmol), and place the reaction system at 90°C Stir in an oil bath for 12 hours. After the reaction, the product is diluted with toluene and passed through a neutral alumina column to remove copper salts. Unpolymerized small molecule monomers and solvents are removed under reduced pressure, and vacuum-dried to obtain poly(tert-butylacrylate-polyethylene) Diol-polyacrylate tert-butyl triblock copolymer, its structural formula is for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com