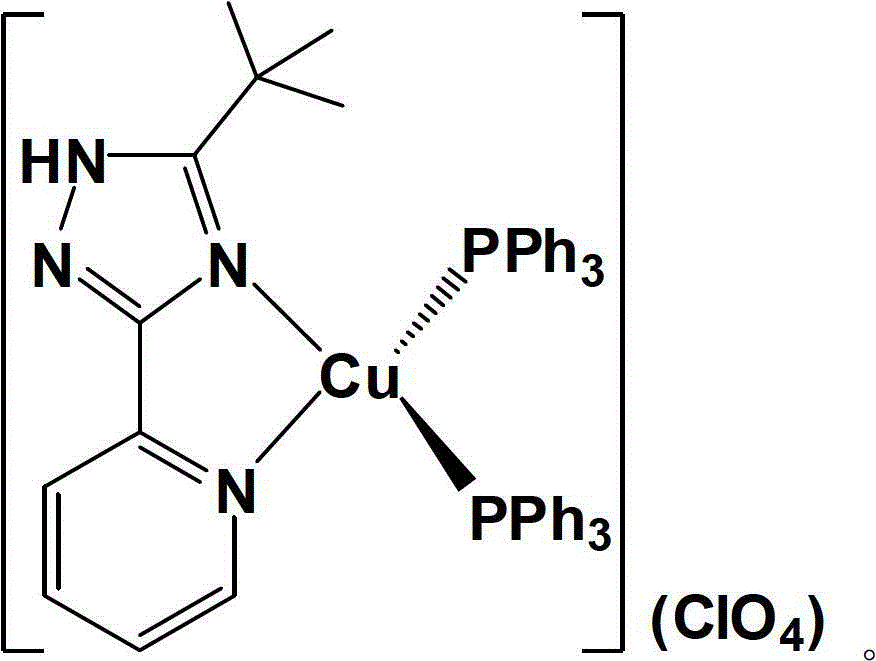
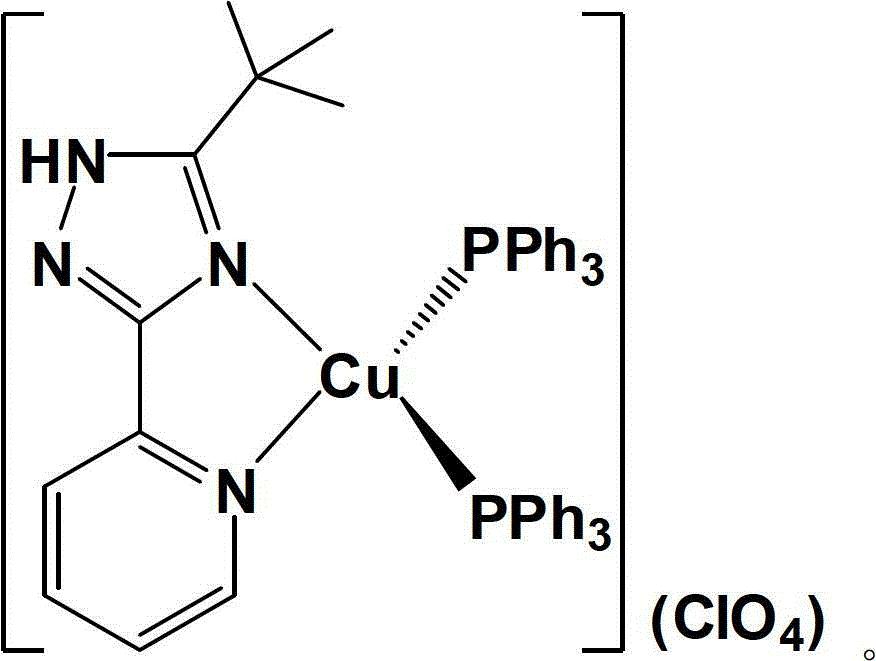
Novel mononuclear copper (I) complex blue light material and preparation method thereof
A technology of blue light materials and complexes, applied in the directions of luminescent materials, copper organic compounds, chemical instruments and methods, etc., to achieve the effects of increasing solubility and film formation, improving luminescence quantum efficiency, and reducing structural relaxation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
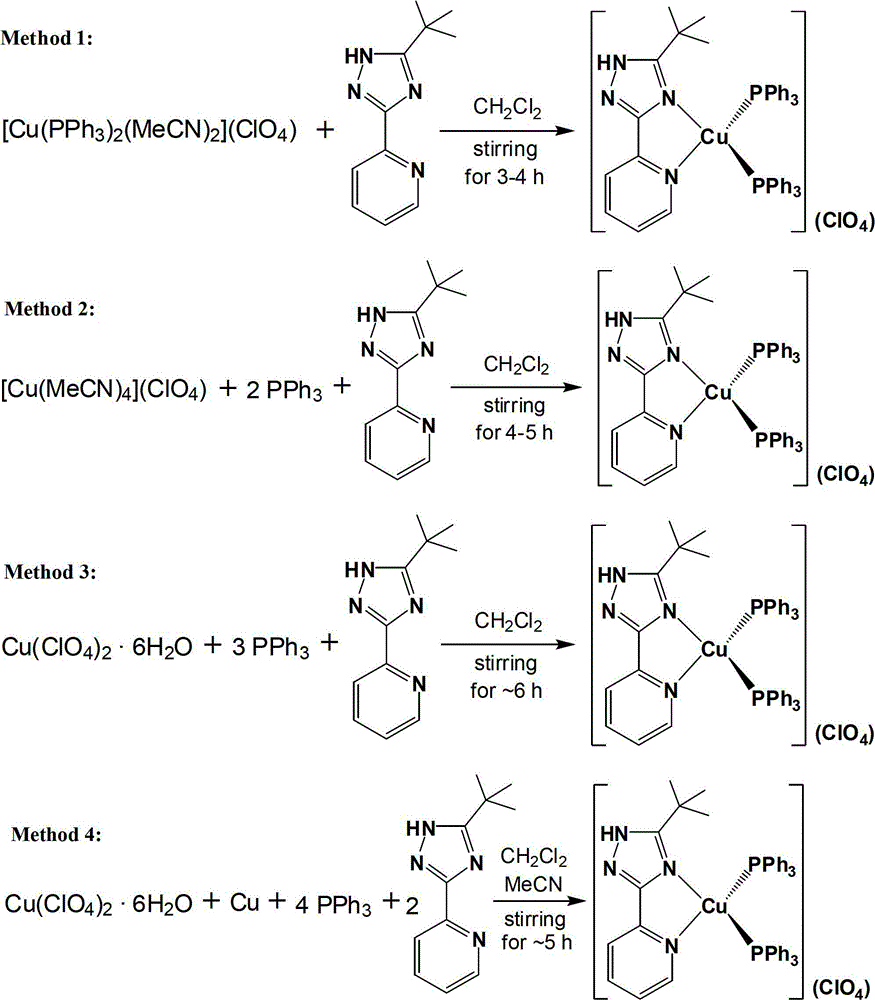
[0033] Under nitrogen atmosphere, [Cu(MeCN) 2 (PPh 3 ) 2 ](ClO 4 ) (385mg, 0.50mmol) and 5-tert-butyl-3-[2-pyridyl]-1,2,4-triazole ligand (106mg, 0.52mmol) (the molar ratio of the two is 1:1.05) Stir the reaction in 50 mL of dichloromethane solvent at room temperature for 4 hours, then evaporate the solvent to dryness on a rotary evaporator, and recrystallize with a mixed solvent of dichloromethane (15 mL)-petroleum ether (90 mL) [volume ratio is 1:6] . The crystalline product obtained by recrystallization was filtered, washed 3-4 times with 60 mL of ether, and dried in vacuo to obtain a pale yellow solid product (378 mg, 0.42 mmol), with a yield of about 84%.
[0034] Proton NMR spectrum (400MHz, DMSO-d 6 , ppm) δ: 14.72(s, 1H), 8.23(d, 1H, J=8.0Hz), 8.04(t, 1H, J=7.6Hz), 7.91(s, 1H), 7.49-7.43(m, 6H ), 7.42-7.33(m, 13H), 7.15-7.10(m, 12H), 1.16(s, 9H)
[0035] NMR phosphorus spectrum (202.3MHz, DMSO-d 6 , ppm) δ: -0.19 (s, PPh 3 )
[0036] Elemental analysis calcul...
Embodiment 2
[0039] Under nitrogen atmosphere, [Cu(MeCN) 4 ](ClO 4 ) (163mg, 0.50mmol) and triphenylphosphine (328mg, 1.25mmol) were stirred and reacted in 50mL dichloromethane solvent for 1 hour to obtain a colorless clear liquid, and then 5-tert-butyl-3 -[2-pyridyl]-1,2,4-triazole ligand (107mg, 0.53mmol) [the molar ratio of the three is 1:2.5:1.05], continue to stir the reaction for 3 hours, and then put it on the rotary evaporator The solvent was evaporated to dryness, and recrystallized with dichloromethane (15 mL)-petroleum ether (90 mL) mixed solvent [volume ratio 1:6]. The crystalline product obtained by filtration and recrystallization was washed 3-4 times with 50 mL of ether, and a light yellow solid product (338 mg, 0.38 mmol) could be obtained after vacuum drying, and the yield was about 76%; The experimental data of elemental analysis are the same as in Example 1.
Embodiment 3
[0041] Under nitrogen atmosphere, copper perchlorate hexahydrate [Cu(ClO 4 ) 2 ·6H 2 O] (185mg, 0.50mmol) and triphenylphosphine (459mg, 1.75mmol) were stirred and reacted in 40mL of dichloromethane solution for 1.5 hours to obtain a transparent and colorless clear liquid, to which 5-tert-butyl-3 -[2-Pyridyl]-1,2,4-triazole ligand (106mg, 0.52mmol) [the molar ratio of the three is 1:3.5:1.05], continue to stir and react for 4.5 hours, and then put it on the rotary evaporator The solvent was evaporated to dryness, and recrystallized with dichloromethane (15 mL)-petroleum ether (90 mL) mixed solvent [volume ratio 1:6]. The crystalline product obtained by recrystallization was filtered, washed 3-4 times with 55 mL of ether, and dried in vacuo to obtain a pale yellow solid product (356 mg, 0.40 mmol), with a yield of about 80%; The experimental data analyzed is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com