Method for preparing atorvastatin calcium intermediate
A technology of atorvastatin calcium and intermediates, which is applied in the field of pharmaceutical organic synthesis, can solve the problems of reduced condensation reaction yield, poor solubility, heterogeneous system, etc. rate increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
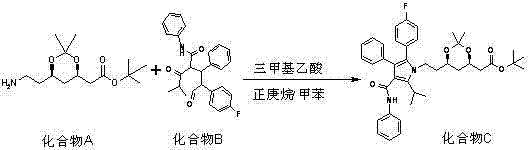
[0033] Under the protection of nitrogen, put 10g of compound A and 18g of compound B into a four-necked flask, add 80g of tetrahydrofuran and 80g of n-butyl ether, stir at room temperature for 15 minutes, add 2g of trimethylacetic acid, and slowly raise the temperature to reflux, After stirring and reflux reaction at 95°C for 6 hours, the purity of Compound C was detected to be 50%, and 0.65g of triethylamine or 1.2g of tert-butylamine was added to continue stirring and reflux reaction for 6 hours, and then the purity of Compound C was detected, and the purity of Compound C was detected to be 78%. , then add 0.65g or 1.2g tert-butylamine triethylamine, and continue the reflux reaction for 6 hours. At this time, the detection purity is greater than 98%, and the reaction is terminated.
[0034] Control the temperature at 60~90℃ and -0.08~-0.096Mpa and concentrate under reduced pressure until no dripping, add 120ml of ethyl acetate and 40ml of water, stir and dissolve, let it stan...
Embodiment 2
[0036] Under the protection of nitrogen, put 10g of compound A and 15g of compound B into a four-necked flask, add 60g of tetrahydrofuran and 60g of n-butyl ether, stir at room temperature for 10 minutes, add 1.5g of trimethylacetic acid, and slowly raise the temperature to reflux , after stirring and reflux reaction at 94°C for 8 hours, the purity of compound C was detected to be 60%, adding 0.35g triethylamine or 0.64g tert-butylamine to continue stirring and reflux reaction for 4 hours, and then continuing to detect the purity of compound C, the purity of compound C was detected to be 80% %, then add 0.35g triethylamine or 0.64g tert-butylamine, and continue the reflux reaction for 4 hours. At this time, the detection purity is greater than 98%, and the reaction is terminated.
[0037] Control the temperature at 60~90℃ and -0.08~-0.096Mpa and concentrate under reduced pressure until no dripping, add 120ml of ethyl acetate and 40ml of water, stir and dissolve, let it stand fo...
Embodiment 3
[0039] Under the protection of nitrogen, put 10g of compound A and 20g of compound B into a four-necked flask, add 100g of tetrahydrofuran and 100g of n-butyl ether, stir at room temperature for 20 minutes, add 2.5g of trimethylacetic acid, and slowly raise the temperature to reflux , after stirring and reflux reaction at 96°C for 4 hours, the purity of compound C was detected to be 55%, adding 0.5g triethylamine or 0.75g tert-butylamine to continue stirring and reflux reaction for 6 hours, and then continuing to detect the purity of compound C, the purity of compound C was detected to be 76% %, then add 0.5g triethylamine or 0.75g tert-butylamine, and continue the reflux reaction for 6 hours. At this time, the detection purity is greater than 98%, and the reaction is terminated.
[0040]Control the temperature at 60~90℃ and -0.08~-0.096Mpa and concentrate under reduced pressure until no dripping, add 120ml of ethyl acetate and 40ml of water, stir and dissolve, let it stand for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com