Rifapentine sustained-released microspheres and preparation method thereof
A technology of rifapentine and sustained-release microspheres, which can be applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. problem, to achieve the effect of good local curative effect, high drug loading, and strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
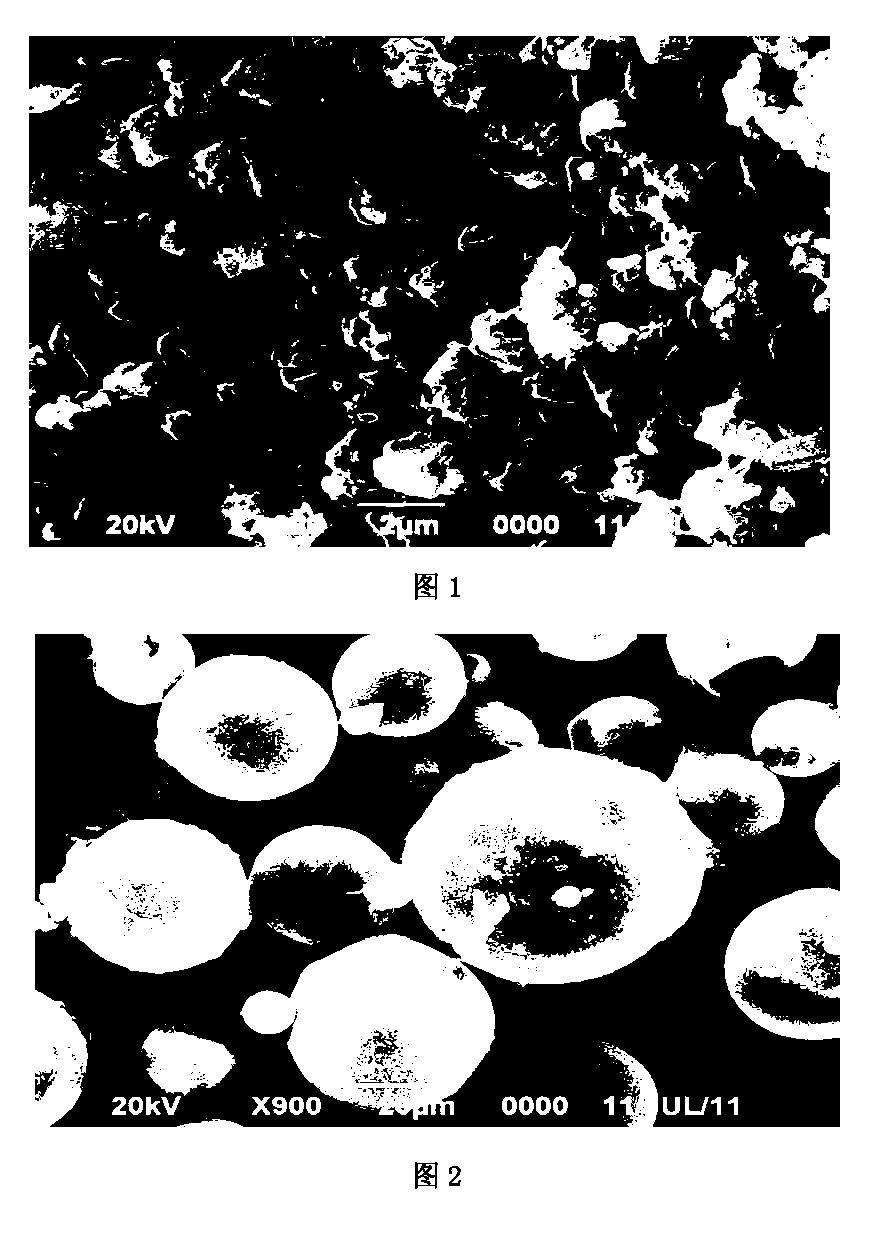
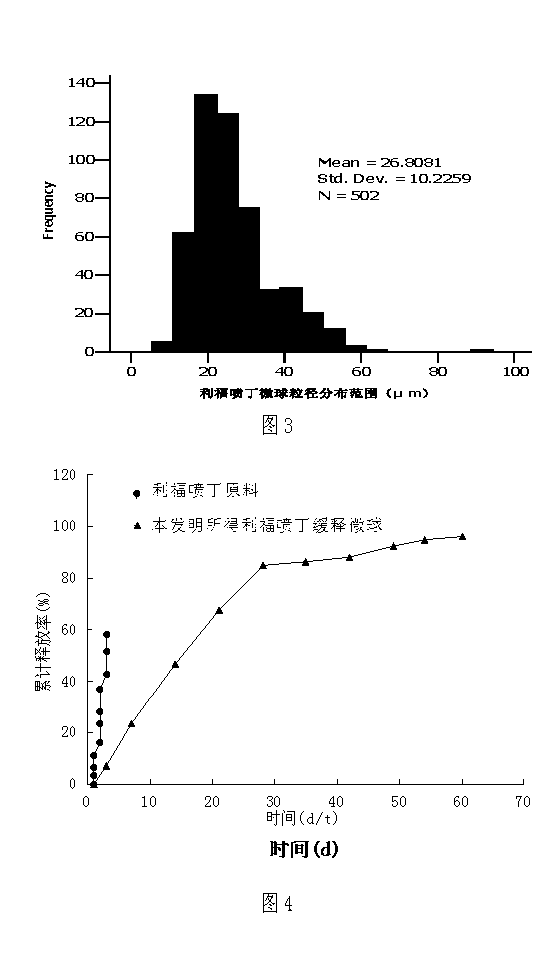
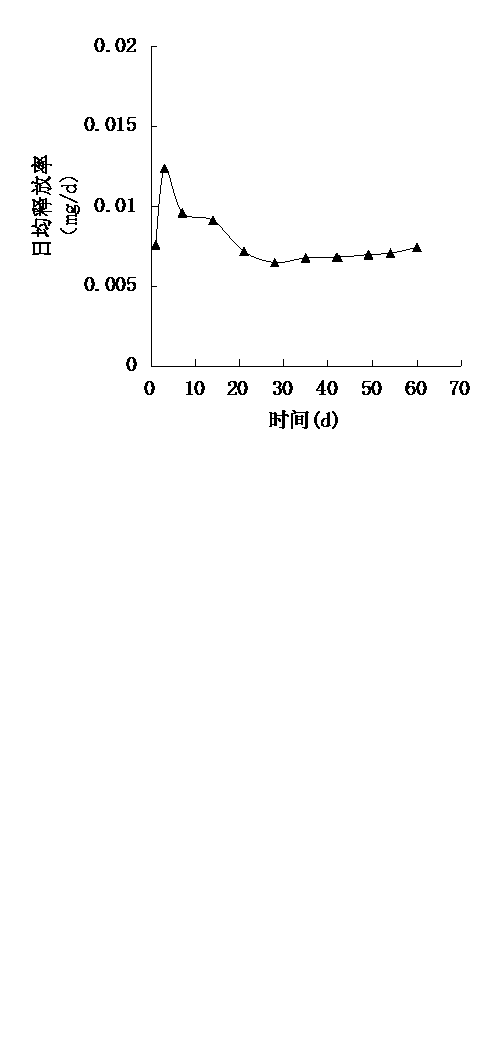
Image
Examples
Embodiment 1
[0024] Example 1, the preparation method of the rifapentine sustained-release microspheres is carried out according to the following steps: the first step, respectively weigh 0.108 g of rifapentine and 0.104 g of polylactic acid with a molecular weight of 10,000 to 50,000, and weigh Dissolve an appropriate amount of gelatin in heated distilled water to prepare a gelatin aqueous solution with a mass concentration of 0.5%. Dissolve polylactic acid in an organic solvent, that is, dichloromethane or trichloromethane. After dissolving in the solution of chloroform, this solution is used as the oil phase, and rifapentine is dissolved in the oil phase to form a colostrum solution; in the second step, the colostrum solution is first ultrasonically homogenized, and the ultrasonically homogenized The colostrum solution is added to the gelatin aqueous solution with a mass concentration of 0.5% to 1.5% under the stirring condition of 1300r / min to 1600r / min, and then emulsified to form a do...
Embodiment 2
[0025] Example 2, the preparation method of the rifapentine sustained-release microspheres is carried out according to the following steps: the first step, respectively weigh 0.103 g of rifapentine and 0.203 g of polylactic acid with a molecular weight of 10000 or 50000, and weigh Dissolve an appropriate amount of gelatin in heated distilled water to prepare a gelatin aqueous solution with a mass concentration of 0.5%. Dissolve polylactic acid in an organic solvent, that is, dichloromethane or trichloromethane. After dissolving in the solution of chloroform, this solution is used as the oil phase, and rifapentine is dissolved in the oil phase to form a colostrum solution; in the second step, the colostrum solution is first ultrasonically homogenized, and the ultrasonically homogenized The colostrum solution is added to the gelatin aqueous solution with a mass concentration of 0.5% or 1.5% under the stirring condition of 1300r / min or 1600r / min, and then emulsified to form a doub...
Embodiment 3
[0026] Example 3, the preparation method of the rifapentine sustained-release microspheres is carried out according to the following steps: the first step, respectively weigh 0.109 g of rifapentine and 0.307 g of polylactic acid with a molecular weight of 30000, and weigh an appropriate amount of gelatin Soluble in heated distilled water, and prepare a gelatin aqueous solution with a mass concentration of 0.5%. Dissolve polylactic acid in an organic solvent, that is, dichloromethane or chloroform, and wait for the polylactic acid to dissolve in an organic solvent, that is, dichloromethane or trichloromethane. After dissolving in the methane solution, the solution is used as the oil phase, and rifapentine is dissolved in the oil phase to form a colostrum solution; in the second step, the colostrum solution is ultrasonically homogenized, and the ultrasonically homogenized colostrum The solution is added to the gelatin aqueous solution with a mass concentration of 0.5% or 1.5% und...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com